The intricate nature of biological structures has long been a source of inspiration for scientific exploration. The idea of self-assembly, akin to assembling an intricate piece of furniture without instructions, mirrors processes observable in nature. Biological entities, from proteins to cell membranes and even entire viruses, demonstrate that complex structures can emerge from relatively simple components. This phenomenon has captured the attention of researchers seeking to replicate similar processes in synthetic environments, particularly within the field of supramolecular chemistry.
Supramolecular chemistry focuses on the organization of large structures from smaller units, promoting the idea that the rules of attraction and interaction can be manipulated to create desired forms. The understanding of how these smaller segments come together or remain separate is crucial for designing “smart materials.” These innovative substances can adapt to external stimuli, such as temperature changes or the introduction of specific chemicals. Despite significant advancements, there are many hurdles that researchers face in fully grasping the complexities of supramolecular interactions.
Insights from Recent Research
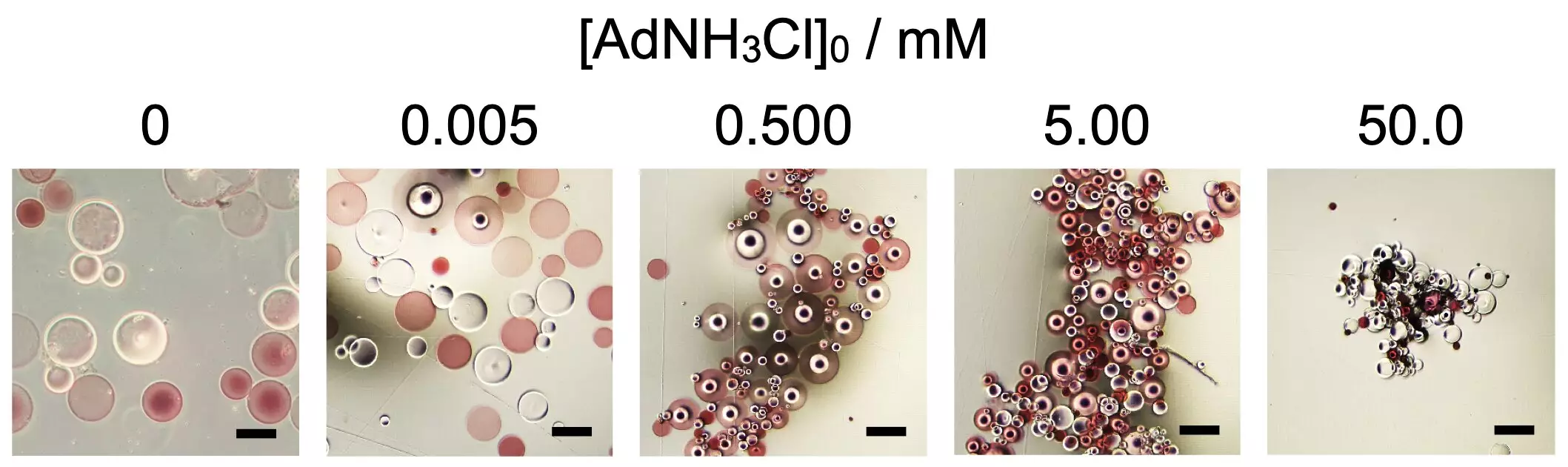
A recent study published in *Scientific Reports* by researchers from Osaka University sheds light on the self-assembly of spherical microparticles made from super absorbent polymers, specifically poly(sodium acrylate). The groundbreaking aspect of this research lies in the manipulation of additives that can influence how these microparticles come together and the overall shape of the resulting structure. Through functionalizing polymer molecules with different chemical agents like β-cyclodextrin and adamantane, the team observed that these particles would only begin to aggregate after a certain concentration of an additive known as 1-adamantanamine hydrochloride was introduced.
This finding is particularly compelling when one considers the pathways through which biological systems operate. Just as proteins fold into complex structures based on interactions among their amino acids—shapes dictated by a range of forces such as hydrogen bonds and hydrophobic interactions—this research indicates that we can achieve a level of control over artificial assemblies by manipulating chemical conditions. The significance of such discoveries extends beyond merely understanding assembly; they have profound implications for how we may create responsive materials in the future.
Applications and Future Directions
The implications of these findings are far-reaching. Akihito Hashidzume, the lead author, highlights that these insights could unravel the evolutionary narratives behind the diverse forms found in living organisms. By establishing a framework for how stimuli like temperature or mechanical forces impact macroscopic assembly shapes, we pave the way for innovations in material science.
Furthermore, such research lays the groundwork for the development of active materials capable of adapting to their environments. This understanding not only enriches supramolecular chemistry but also informs fields ranging from biotechnology to materials engineering. With ongoing exploration, we may soon harness these principles to create transformative applications in health, sustainability, and beyond. The promise of self-assembly beckons us to look more closely at the mesmerizing patterns of nature, where the smallest interactions can yield the most substantial results.

Leave a Reply