Proteins are the building blocks of life, intricately involved in myriad biological processes, from cellular growth to complex metabolic pathways. Among these, the protein myo-inositol-1-phosphate synthase (MIPS) has garnered significant attention due to its pivotal role in inositol production, a compound crucial for various physiological functions. Recent groundbreaking research conducted by an interdisciplinary team from Martin Luther University Halle-Wittenberg (MLU) and the National Hellenic Research Center in Greece has provided new insights into how MIPS alters its internal structure upon activation. This transformative ability of MIPS sheds light on the broader implications for protein functionality and the potential for novel therapeutic advancements.
In the context of metabolic pathways, MIPS serves as a critical enzyme in synthesizing inositol, often dubbed vitamin B8. While the body generates this compound, it is essential for numerous biochemical functions, including cell signaling and fat metabolism. An essential tenet of protein research posits that a protein’s structure directly dictates its function. Disruptions at any structural point can severely hinder its operational capacity, potentially leading to various diseases. This fundamental principle emphasizes the importance of understanding MIPS and its structural dynamics, especially since many proteins, including MIPS, exhibit a lack of stable conformation and are sensitive to environmental changes.
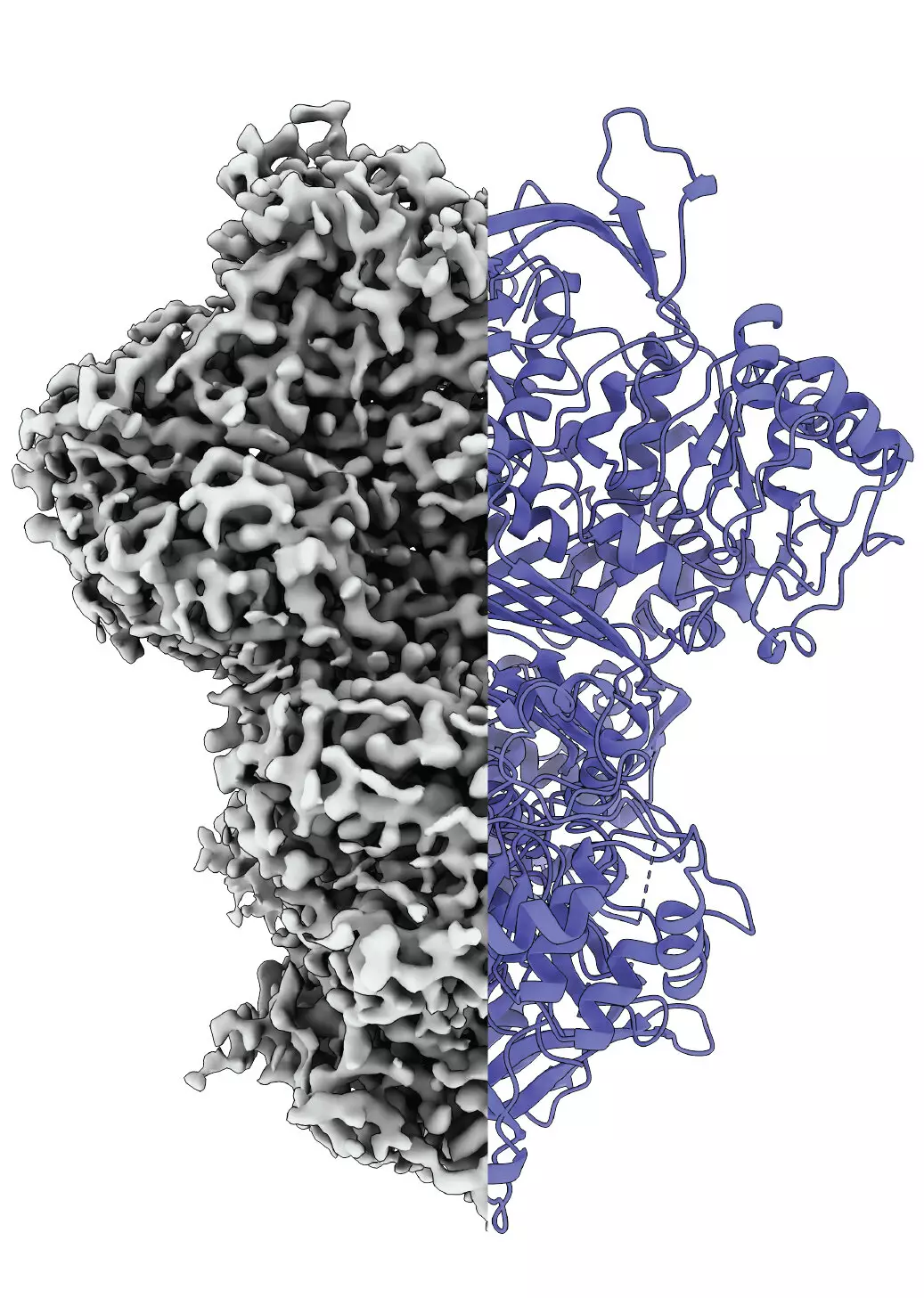
The challenges posed by such disordered proteins are compounded by traditional analytical techniques that typically isolate proteins from their native surroundings, risking the loss of crucial information regarding their natural behaviors and interactions. Professor Panagiotis Kastritis, a leading biochemist involved in the MIPS research, highlights the importance of studying proteins under conditions that closely mimic their physiological environment to grasp their true function. The recent study did precisely that by examining samples derived from Thermochaetoides thermophila, a fungal model organism.
Utilizing advanced cryo-electron microscopy, the researchers observed MIPS in action, uncovering its existence in three distinct states: disordered, ordered, and an intermediate state. This third state is particularly intriguing, as its function remains unclear. It may facilitate water absorption to prepare for subsequent reactions, indicating the complexity of protein behavior and interaction. Kastritis poses an open question regarding the necessity of this intermediate state, inviting further exploration into the mechanics of protein activation.
The implications of these findings extend beyond MIPS alone. Taking a broader perspective, the researchers investigated over 340 proteins related to MIPS, categorized as isomerases. The analysis revealed a pattern suggesting that many of these proteins likely share similar structural behaviors and activation mechanisms. This revelation could significantly streamline future protein research, providing clearer pathways to understanding how proteins operate and respond in various biochemical contexts.
The significant advances in comprehending the structural dynamics of proteins like MIPS not only enhance the field of basic scientific research but also pave the way for innovative therapeutic strategies. An understanding of metabolic pathways and the specific roles of enzymes can lead to the identification of new targets for drug development, offering potential solutions for various health issues stemming from metabolic disturbances. As Kastritis aptly notes, the research marks an important first step in unveiling the complexities of metabolic processes and how they can be manipulated for therapeutic benefits.
The work conducted by the MLU and National Hellenic Research Center teams signifies a crucial leap forward in protein research. By uncovering the dynamic restructuring of MIPS and suggesting parallels with other isomerases, they have opened new avenues for inquiry into protein functionality. As research continues to evolve, further insights will undoubtedly emerge, enhancing the scientific community’s understanding of life at the molecular level and offering promising prospects for innovative healthcare solutions.

Leave a Reply