As the world actively pursues sustainable energy solutions, the significance of efficient catalysts in energy conversion processes cannot be overstated. Among various chemical reactions, the oxygen evolution reaction (OER) stands out as a pivotal component in water splitting, which is essential for hydrogen production. Traditional strategies for facilitating this reaction have often relied on precious metals, making the development of cost-effective and robust alternatives a pressing necessity. Recent advancements in the realm of electrocatalysis have introduced innovative materials that promise not only enhanced functionality but also economic viability.
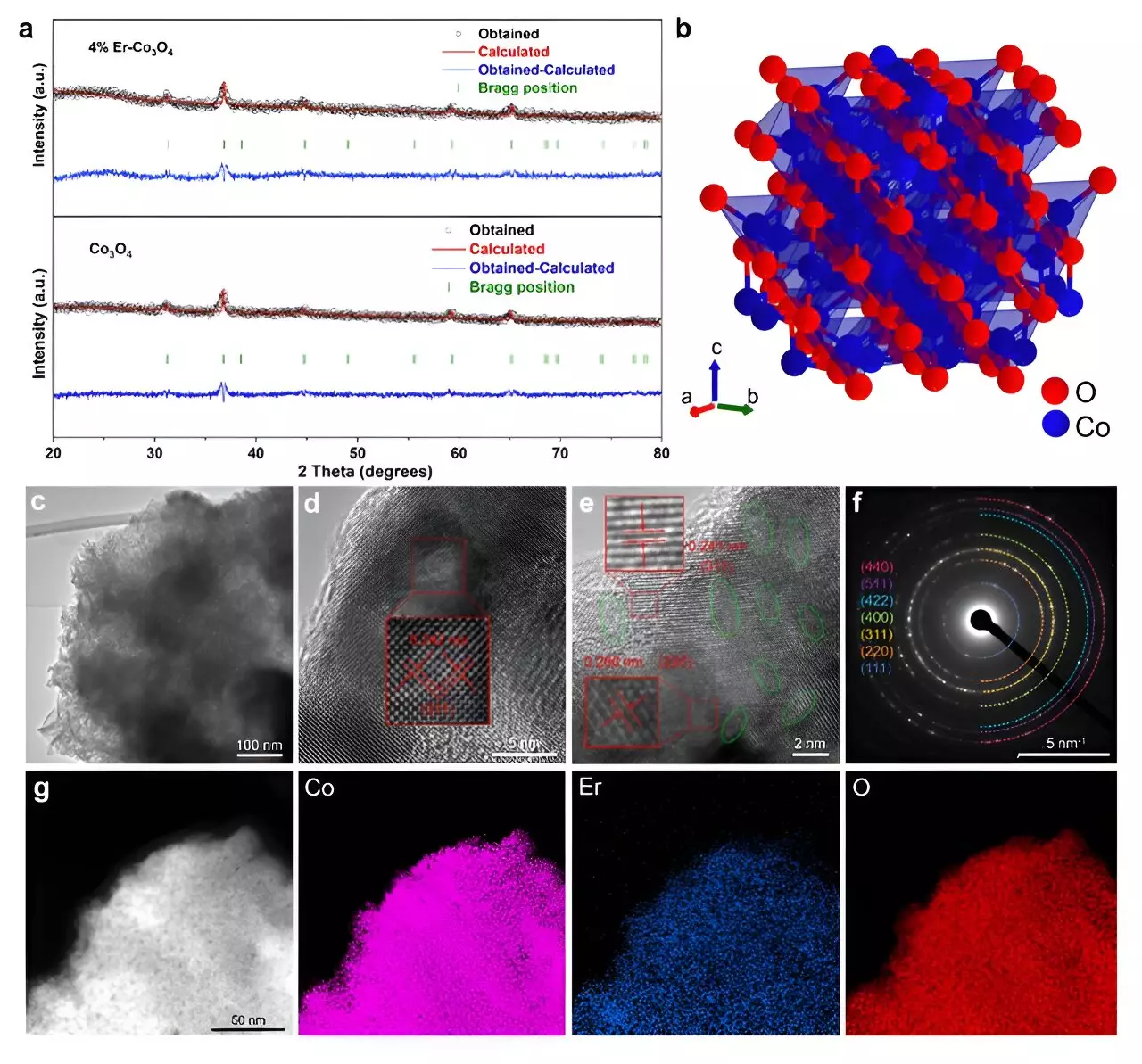
A striking breakthrough has emerged from a team of researchers who explored the incorporation of erbium (Er), a rare earth element, into cobalt oxide (Co3O4) catalysts. Their work was published in the respected journal ACS Catalysis, garnering attention as the Editors’ Choice. The innovation of creating a cobalt oxide catalyst doped with erbium demonstrates the potential to significantly surpass the performance metrics of many current non-precious metal catalysts. By integrating erbium into cobalt oxide, the research presents a viable alternative to traditional noble metal catalysts like iridium.
Tianyi Wang, a postdoctoral researcher affiliated with Tohoku University’s Advanced Institute for Materials Research, emphasizes the significance of this development. “Our findings indicate that even a modest amount of erbium can lead to notable enhancements in both the activity and longevity of the catalyst,” he states. The results showcased an outstanding overpotential of 321 mV at a current density of 10 mA cm² and maintained this performance for over 250 hours. This remarkable consistency places erbium-doped cobalt oxide on par with, and sometimes outperforming, much pricier iridium-based alternatives.
Mechanistic Insights into Enhanced Performance
What sets apart the erbium-doped Co3O4 catalyst is not merely its composition but the fundamental changes it brings to the catalyst structure at the atomic level. The research team employed sophisticated analytical techniques, including microkinetic modeling and density functional theory calculations, to dissect the underlying mechanics of the OER process. The introduction of erbium leads to an increase in active sites and enhances the formation of oxygen vacancies, which are vital for facilitating more efficient reactions.
By refining the ratio of Co3+ to Co2+ ions in the catalyst structure, the researchers effectively capitalized on the creation of these oxygen vacancies. This shift is analogous to adding additional lanes to a busy highway, allowing a more substantial flow of “traffic”—in this case, the reaction intermediates necessary for the OER. Such analogies serve to clarify the complex interplay of electronic and structural factors that govern the catalyst’s performance.
Hao Li, an associate professor involved in the research, elaborates, “The unique modification brought about by Er doping is akin to optimizing traffic flow. The extra lanes, represented by oxygen vacancies, expedite the movement of oxygen intermediates during the reaction, thereby enhancing overall efficiency.” This innovative approach melds electronic and structural optimizations, paving the way for a new benchmark in catalyst design.
The implications of this research extend beyond immediate performance gains. By demonstrating a pathway toward economically viable and sustainable catalysts, the study opens doors for further exploration into non-precious metal doping strategies. The research team is poised to investigate the scope of utilizing other readily available metals in combination with cobalt oxide, further pushing the boundary of catalyst engineering.
The exploration of erbium-doped cobalt oxide signifies a move toward a greener and more sustainable energy future. Continued research in this area will not only contribute significantly to the field of electrocatalysis but also aid in addressing global energy challenges. By prioritizing affordability and sustainability, the development of these innovative materials could mark a critical turning point in our approach to energy conversion technologies. As the search for efficient and cost-effective solutions continues, the work of these researchers serves as a shining example of how innovative thinking can yield transformative advancements in science and technology.

Leave a Reply