As global concerns about climate change escalate, one of the central issues is the continuous accumulation of carbon dioxide (CO2) in our atmosphere. Emissions stem from various sources, including electricity generation, transportation, and industrial processes. To address this pressing problem, scientists are tirelessly seeking innovative methods to not only reduce these emissions but also recycle CO2 into useful products. Among various strategies explored, electrochemical reduction stands out as a promising solution. This process utilizes electrical energy to transform recaptured CO2 into commercially viable products, such as methanol and ethanol, therefore potentially contributing to a circular carbon economy.
The Quest for Efficient Catalysts
Despite its potential benefits, one significant challenge in the field of electrochemical reduction is the quest for efficient catalysts. Effective catalysts must accelerate the chemical reactions involved in CO2 conversion without demanding excessive energy, which can hinder their practical application on a large scale. Researchers are driven by the need to improve catalytic efficiency while minimizing energy input, making the development of novel catalysts crucial for real-world implementation.
A recent breakthrough, led by scientists at the U.S. Department of Energy’s Brookhaven National Laboratory in collaboration with Yale University and UNC Chapel Hill, has shown promise in addressing this challenge. Their research highlighted a new way to enhance catalytic speed—an advance reported in the August 2024 edition of the Journal of the American Chemical Society.
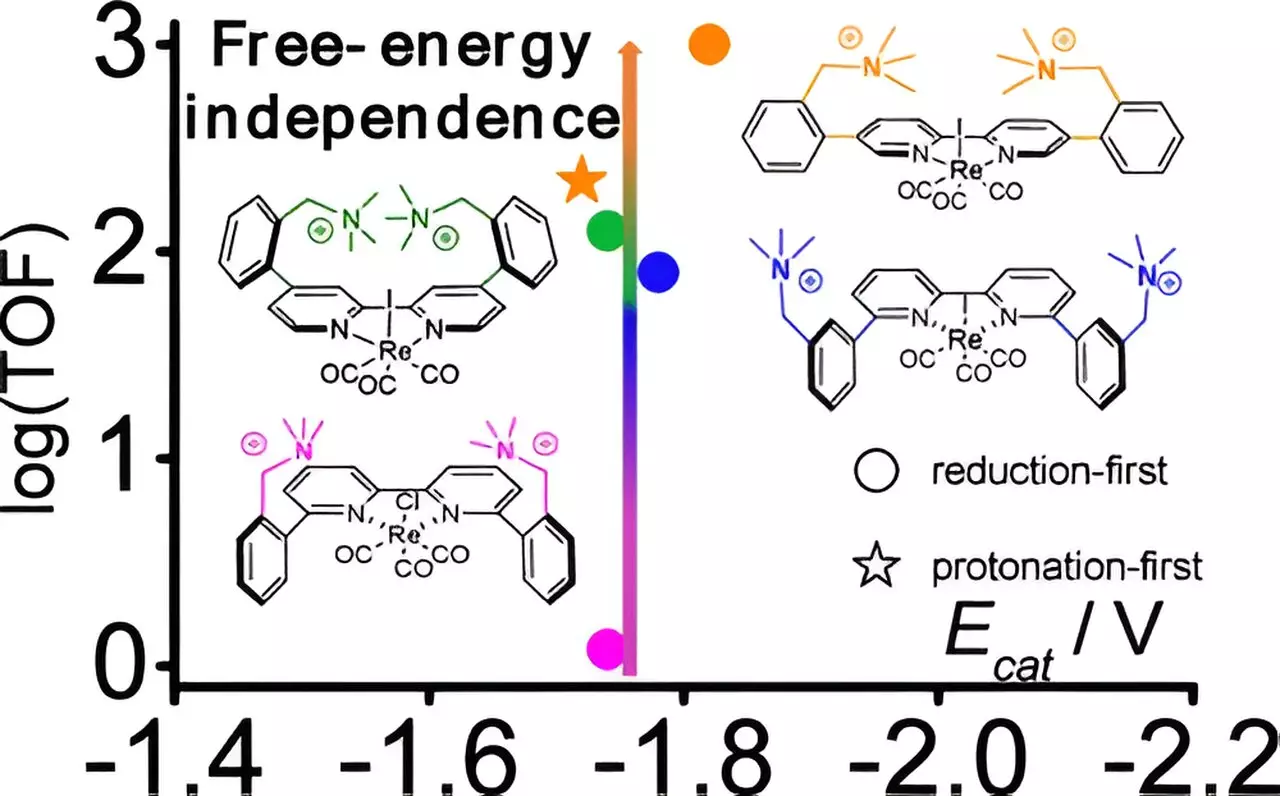
The study focused on improving an existing rhenium-based catalyst. Researchers realized that the arrangement and distance between various components of the catalyst could dramatically affect its performance. By introducing positively charged molecules, or cations, they designed three new versions of the catalyst, strategically altering the distance between the rhenium center and these cations. This research uncovered a remarkable increase in catalytic activity, reportedly by a staggering factor of 800, all while requiring much less electrical energy than traditional methods.
As highlighted by Gerald Manbeck, one of the principal researchers, achieving such a significant enhancement in the catalytic rate is not just an incremental advancement; it represents a critical shift that could inform future catalyst design. This point reinforces the importance of refining the geometric structure of catalysts to unlock previously unattainable efficiencies.
The success of this research was bolstered by the application of computational chemistry. This approach allowed researchers to simulate and predict the behavior of the newly designed catalysts and to understand the stabilizing effects of cations on the later stages of the catalytic reaction. Computational tools provided insight into how these structural changes could create a low-energy pathway, a significant discovery in the context of rhenium-based catalysts, which typically do not exhibit such properties.
Utilizing advanced resources like the Center for Functional Nanomaterials and the Scientific Data and Computing Center at Brookhaven, the team was able to achieve a detailed understanding of the reaction mechanisms at play. This understanding is vital for paving the way toward future research focused on optimizing existing catalytic frameworks.
To validate their findings, the research group employed various experimental methods, including cyclic voltammetry and infrared spectroelectrochemistry. The combination of these techniques offered comprehensive data on the energy characteristics and reaction rates and provided valuable insights into the structural changes occurring throughout the reactions. Notably, their use of a novel apparatus designed for observing minute chemical changes near the electrode-solution interface proved essential in comprehensively analyzing the catalysts’ behavior.
Such meticulous methodological approaches serve not only to strengthen the validity of the research findings but also contribute to the broader scientific discourse surrounding catalyst development and CO2 reduction techniques.
Looking ahead, the research team envisions adding another layer to their work by integrating semiconductor-based light absorbers, such as silicon, into their catalytic system. This integration may allow incoming sunlight to provide partial energy to drive the catalytic process, further reducing reliance on direct electrical energy. This avenue of research aligns with the broader mission of projects like CHASE, aimed at developing photoelectrodes that effectively harness solar energy for chemical conversions, enhancing the sustainability of CO2 reduction technologies.
This ongoing investigation not only highlights an essential advancement in catalyst technology but also underscores the collaborative efforts needed to tackle climate change. As breakthroughs like these emerge, the prospects for effective CO2 mitigation and the creation of sustainable fuels become more tangible, representing a hopeful step in addressing one of the most significant global challenges of our time.

Leave a Reply