The global demand for sustainable energy sources has intensified due to the pressing challenges of climate change and the depletion of fossil fuels. Among the frontrunners in this quest for clean energy, hydrogen energy has gained significant traction. Renowned for its high calorific value and low carbon emissions, hydrogen is poised to play a pivotal role in mitigating the energy crisis. A particularly promising method of harnessing hydrogen is through water splitting, an electrochemical process that faces key barriers in efficiency. One of the main culprits in this inefficiency is the sluggish kinetics associated with the oxygen evolution reaction (OER) that occurs at the anode during the water-splitting process. Consequently, the quest for efficient catalysts for the OER has become a focal point of research.
Catalysts are vital components in enhancing the rate of chemical reactions without undergoing permanent changes. In the context of water splitting, the efficiency of these catalysts can greatly influence the viability of hydrogen production as a mainstream energy source. Recent advancements in catalysis often highlight single-atom catalysts (SACs) as a groundbreaking solution. These SACs possess the unique capability of optimizing reaction paths thanks to their atomic structure. However, until recently, the development of effective SACs has been limited by challenges in optimizing catalyst designs that simultaneously maximize performance and stability.
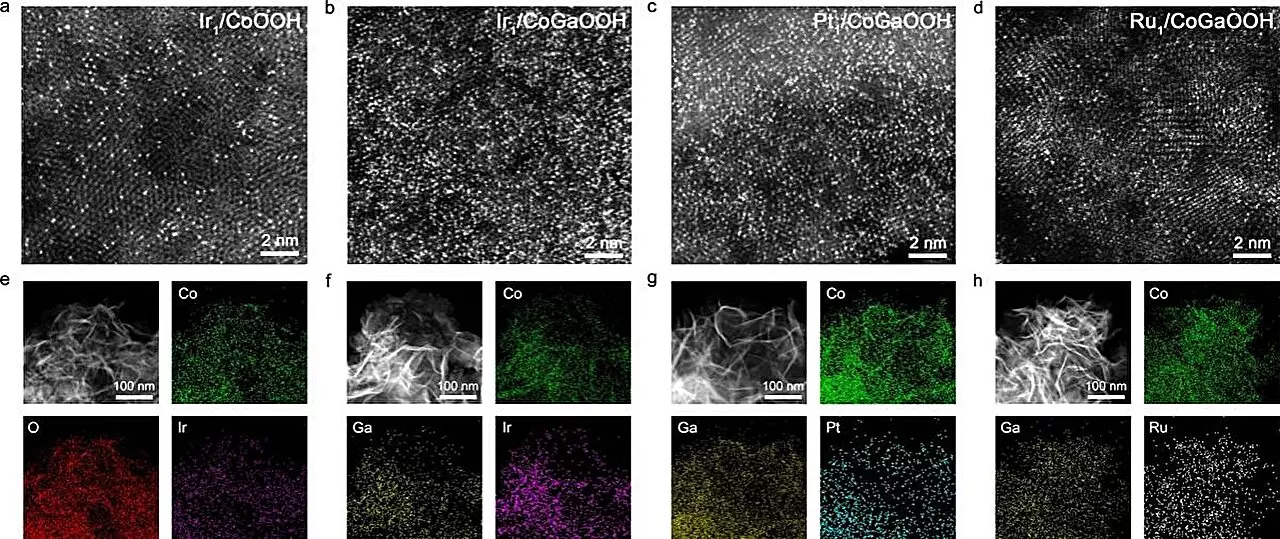
Research spearheaded by Prof. Bao Jun from the University of Science and Technology of China has introduced a breakthrough in this arena, specifically focusing on a high-density iridium (Ir) single-atom catalyst supported by cobalt-based oxides. This catalyst’s remarkable performance can be attributed to synergetic interactions between neighboring single atoms, a phenomenon that enhances the catalyst’s functionality and paves the way for more efficient hydrogen generation.
The pivotal aspect of this research is the concept of neighboring synergetic interactions among single atoms. Typically, the efficacy of single-atom catalysts is contingent upon their surface density—an increase in single-atom density leads to closer proximity among the atoms. This close interaction fosters a novel synergy that optimizes the adsorption characteristics of reaction intermediates, consequently boosting the overall catalytic performance. By ingeniously introducing gallium (Ga) atoms into the cobalt oxide lattice, the research team was able to manipulate the electronic structures of the anchoring sites, thus successfully synthesizing a series of high-density SACs.
The efficacy of their catalyst, known as Nei-Ir1/CoGaOOH, was evaluated through rigorous experimentation, revealing a commendable overpotential of only 170 mV at a current density of 10 mA cm-2. What is particularly impressive is the catalyst’s durability; it maintained stable operation for over 50 hours at a current density of 1 A cm-2 in alkaline environments, showcasing potential for industrial applications.
The revelations from this study extend beyond just improved energy conversion; they unearth the fundamental mechanisms underpinning the enhancement of catalyst performance through neighboring synergetic interactions. This insight has far-reaching implications. As researchers aim to design next-generation catalysts for various electrochemical processes, such understanding will be crucial in developing more efficient and stable materials.
The exploration of high-density single-atom catalysts, particularly the advancements made with iridium-based systems, signifies a substantial leap forward in the field of hydrogen energy. By addressing the kinetic limitations of the oxygen evolution reaction through innovative catalyst designs, this research marks a critical step toward achieving high-efficiency hydrogen production. The successful integration of neighboring synergetic interactions serves not only as a model for future catalyst development but also as an invitation for interdisciplinary collaboration, enhancing our collective efforts to achieve sustainable energy solutions. As we stand at the precipice of a new era in energy production, the findings from this study remind us of the potential that lies in innovative approaches to catalysis and the vital role these technologies will play in shaping our energy landscape.

Leave a Reply