Recent advancements in the field of chemistry have unveiled the intricate mechanisms underlying the interactions of cobalt(III)-based complexes with nitrile compounds. Led by Professor Jaeheung Cho from the Department of Chemistry at UNIST, a dedicated research team has published compelling findings in the Journal of the American Chemical Society. This study not only broadens the horizon for biomimetic compounds but also underscores the significance of metal spin states in influencing chemical reaction dynamics. Such insights could be pivotal in the development of new pharmaceuticals.
The research centered on the activation of nitriles, a class of compounds frequently utilized in various drug formulations and pesticides. However, their inherently low reactivity often hampers effective utilization in chemical reactions. By employing cobalt(III) as a catalyst, the team navigated the complexities of nitrile interactions, revealing that even minor alterations in the metal’s properties could exert considerable effects on reaction rates and products. This correlational insight prompts a reshaping of traditional perspectives on catalyst efficiency in organic chemistry.
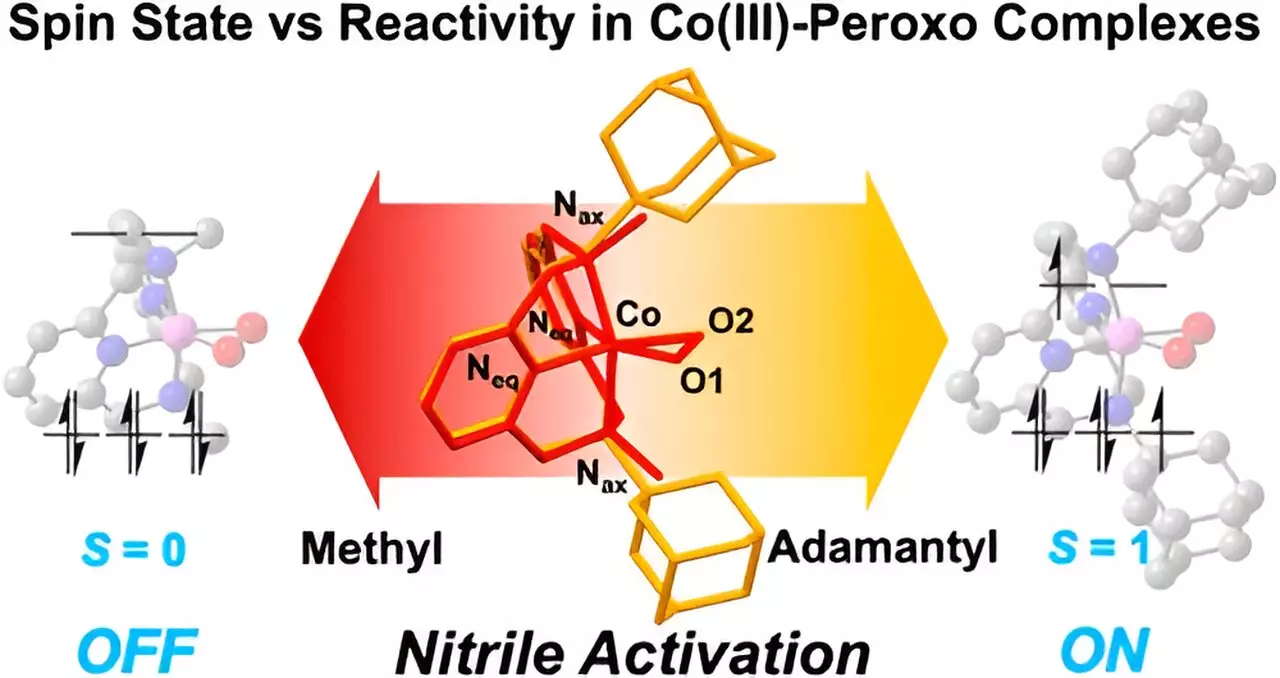
A notable aspect of this study is the use of the Macrocyclic Pyridinophane System, allowing fine-tuning of cobalt compound structures. By manipulating these structures, researchers discerned that compounds equipped with larger adamantyl groups exhibited markedly improved reactivity towards nitriles. Conversely, those with petite methyl groups failed to initiate significant reactions. This variance highlights a crucial phenomenon: the influence of metal spin states, which are directly affected by the size of functional groups in the compound. The study emphasizes how configurations at the molecular level can dictate reactivity, advancing our understanding of chemical interactions.
The research further delves into the promising realm of cobalt(III)-peroxo species. These newly synthesized varieties possess versatile spin states that enhance their ability to react with nitriles at ambient temperatures. The resultant compounds demonstrate potential as anticancer agents, opening up avenues for innovative drug formulations. First author Seonghan Kim articulated the significance of their findings, noting the profound connection between ligand architecture and nitrile reactivity. By manipulating the three-dimensional arrangement of ligands, researchers successfully tailored the chemical behavior of these complexes.
As pharmaceutical research increasingly pivots towards biomimetic approaches, the implications of this study are immense. The findings not only elucidate foundational chemical principles but also hint at practical applications in the synthesis of emerging drugs. By presenting substantive evidence that cobalt(III)-based complexes can enhance nitrile reactivity, the research provides a vital resource for future investigations aiming to design more effective therapeutic agents. The evolution of metal complexes in drug design signifies a promising frontier, awaiting further exploration and development.

Leave a Reply