In the realm of atmospheric chemistry, the study of sulfur compounds has long been a complex and intriguing subject. Recent groundbreaking research from the Leibniz Institute for Tropospheric Research (TROPOS) in Leipzig has illuminated the elusive nature of sulfurous acid (H₂SO₃) within our atmosphere, a feat considered nearly impossible until now. Published in the esteemed journal Angewandte Chemie, this research challenges conventional wisdom that has deprived H₂SO₃ of recognition in its isolated form, primarily due to the belief that it could not exist outside of aqueous solutions.
Historically, textbooks have posited that H₂SO₃ could form in vigorous aqueous solutions of sulfur dioxide (SO₂), yet attempts to verify its presence in these conditions through various spectroscopic methods have proven futile. Instead, scientists could only detect its related compounds, bisulfite (HSO₃⁻) and sulfite (SO₃²⁻). The only known instance of H₂SO₃ detection prior to this study dates back to 1988, when a team led by Helmut Schwarz achieved this through an innovative in-situ method inside a mass spectrometer. However, even this early detection was short-lived, raising questions about the chemical stability and significance of sulfurous acid in the atmosphere.
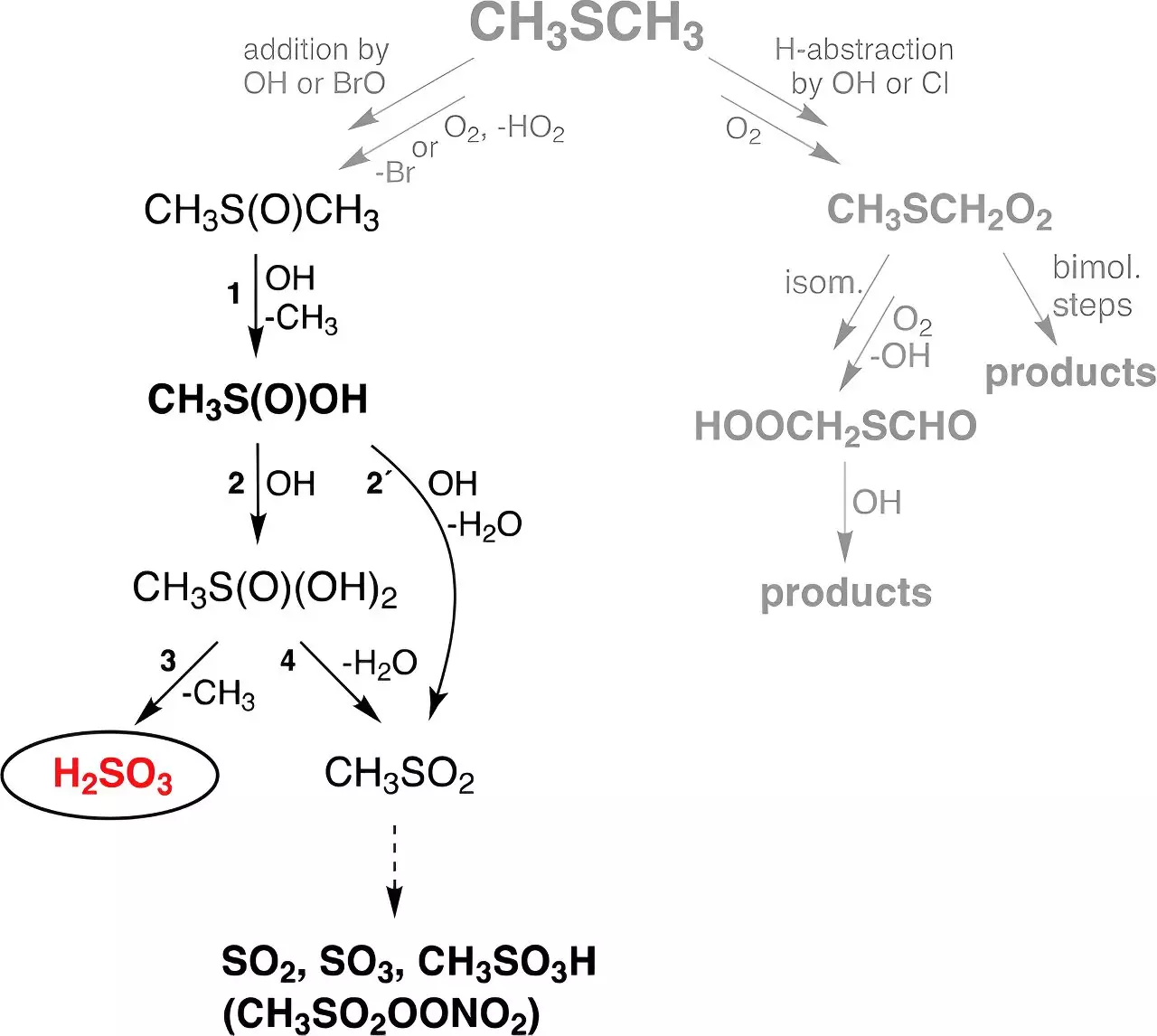
The latest TROPOS endeavor utilized advanced experimental techniques to explore an alternative pathway for H₂SO₃ formation via dimethyl sulfide (DMS), a gas produced largely from marine biological activities. DMS is a significant biogenic sulfur source, contributing an estimated 30 million tons of sulfur to the atmosphere every year. The researchers meticulously investigated how DMS reacts in the presence of hydroxyl (OH) radicals—formed mainly from ozone interactions with water vapor under UV irradiation—leading to the ground-breaking discovery of H₂SO₃ as a gas-phase product.
The experimental setup employed flow reactors that simulated atmospheric conditions, allowing scientists to witness the formation and stability of H₂SO₃. Their findings revealed that sulfurous acid could persist in the gas phase for approximately half a minute, a significant discovery given that longer stability periods had not been thoroughly explored due to limitations in previous experimental arrangements. The implications of this stability suggest that H₂SO₃ might play a far more critical role in atmospheric chemistry than previously thought, potentially influencing complex chemical processes in ways that remain to be elucidated.
What sets this research apart is not just the experimental validation of H₂SO₃’s existence but also the considerable yield observed, which surpassed theoretical expectations. According to Dr. Torsten Berndt, the lead researcher at TROPOS, the spectrometer signals captured during the experiments were “impressive” for a compound that was long presumed to be non-existent. Remarkably, the research incorporated this newly identified reaction pathway into global chemistry-climate models, projecting that around 8 million tons of H₂SO₃ are being produced annually through these processes.
This revelation is vital as it suggests that the formation of H₂SO₃ from DMS is remarkably more prolific—about 200 times—than the well-studied formation of sulfuric acid (H₂SO₄) in the atmosphere. The newfound understanding of sulfur cycling emphasizes the necessity of reevaluating existing atmospheric models to incorporate these dynamics, as they are crucial for accurately representing sulfur’s role in climate and environmental change.
Despite these advancements, the research leaves many accompanying questions unanswered. The stability of H₂SO₃ in relation to its interactions with trace atmospheric gases remains largely enigmatic, particularly the reaction dynamics with water vapor. Dr. Berndt emphasizes that current knowledge surrounding H₂SO₃’s atmospheric lifetime must be deepened through continued experimentation. The complexity of atmospheric reactions demands refined methodologies and improved detection techniques, which can provide insight into compounds previously shrouded in theoretical ambiguity.
The advent of high-sensitivity detection tools—such as the mass spectrometer utilized in this research, capable of discerning a single molecule among a staggering collection of 10 quadrillion molecules—marks a pivotal milestone in atmospheric chemistry research. These innovations not only bring us closer to understanding sulfur compounds but also advocate for a renewed focus on exploring the intricate dance of chemistry that influences global climate systems.
The discovery of sulfurous acid in the atmosphere is a compelling reminder of the continuous evolution of scientific inquiry. As researchers peel back the layers of complexity surrounding atmospheric chemistry, they uncover previously obscured pathways that will enhance our understanding of environmental processes. The interplay between DMS and sulfurous acid demonstrates that the field of atmospheric science remains ripe with untapped knowledge, waiting for dedicated researchers to illuminate its mysteries in the years to come.

Leave a Reply