Recent advancements in molecular biology have brought to light the intricate processes that underline the fundamental role of ribosomes in protein synthesis. At the forefront of this research are the scientists at the University of Tsukuba, who have ingeniously developed a robust model that mimics the internal environment of ribosomes, the cellular structures responsible for amino acid assembly into proteins. Utilizing cutting-edge computer simulations, these researchers have made significant strides in uncovering how the ribosome tunnel—a conduit for nascent proteins—plays a critical role in shaping their final three-dimensional (3D) structures.
The ribosome tunnel is where the magic happens; it is within this tubular passage that newly synthesized proteins begin to fold and take on their necessary configurations. Disturbingly, until now, the exact dynamics of protein structure formation during translation—a critical phase of protein synthesis—have remained enigmatic. The University of Tsukuba’s research seeks to fill this gap by providing a detailed analysis of the ribosome tunnel’s structure and its chemical properties. These aspects are pivotal for understanding how proteins can begin their critical folding process while they are still embedded in the ribosomal environment.
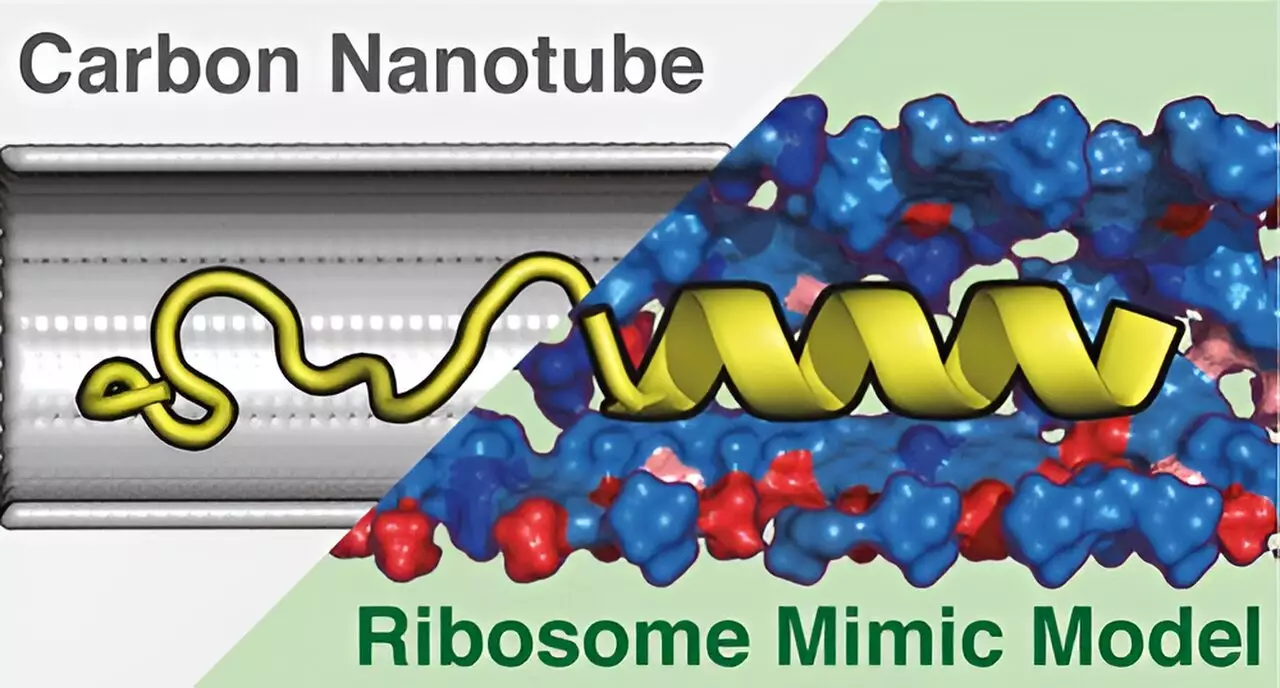
In their pursuit to clarify these mechanisms, the research team has introduced the Ribosome Environment Mimicking Model (REMM). This cylindrical model is not only designed to replicate the dimensions of ribosome tunnels but also to reflect their unique chemical characteristics. To determine the efficacy of their model, the team juxtaposed REEMM against a more traditional model constructed from carbon nanotubes (CNTs). While CNTs may replicate the tunnel’s diameter, they fall short in simulating its chemical nuance, significantly impairing the model’s accuracy.
The crux of the researchers’ experimentation involved using molecular dynamics simulations to investigate how proteins behave within both the REMM and CNT models. The findings were illuminating: REMM offered a markedly closer approximation of experimentally observed protein structures compared to the CNT framework. This key differentiation underscores the importance of accounting for chemical diversity during protein synthesis, as it emerged as a substantial factor influencing the accuracy of protein conformation replication.
The implications of this study extend beyond mere academic curiosity; they could redefine how we approach the understanding of protein synthesis in live cells. By refining the REMM further, researchers aim to illuminate the sophisticated folding processes that occur within ribosomes, potentially unlocking new avenues in biotechnology and pharmaceutical development. This innovative work continues to pave the way for a deeper understanding of cellular mechanisms, conquering the remaining barriers in cellular biology one simulation at a time.

Leave a Reply