The investigation conducted by scientists from China sheds light on the intricate process of short peptide chains aggregating together. This research, recently published in JACS Au, delves into the interactions, folding, and functions of short proteins known as peptides. The implications of these findings are far-reaching, impacting medicine, material science, and biotechnology.
Peptides, consisting of short chains of amino acids, play crucial roles in the human body. They contribute to building structures, facilitating chemical reactions, and supporting the immune system. The functionality of a protein is determined by how its amino acids interact and form a three-dimensional structure.
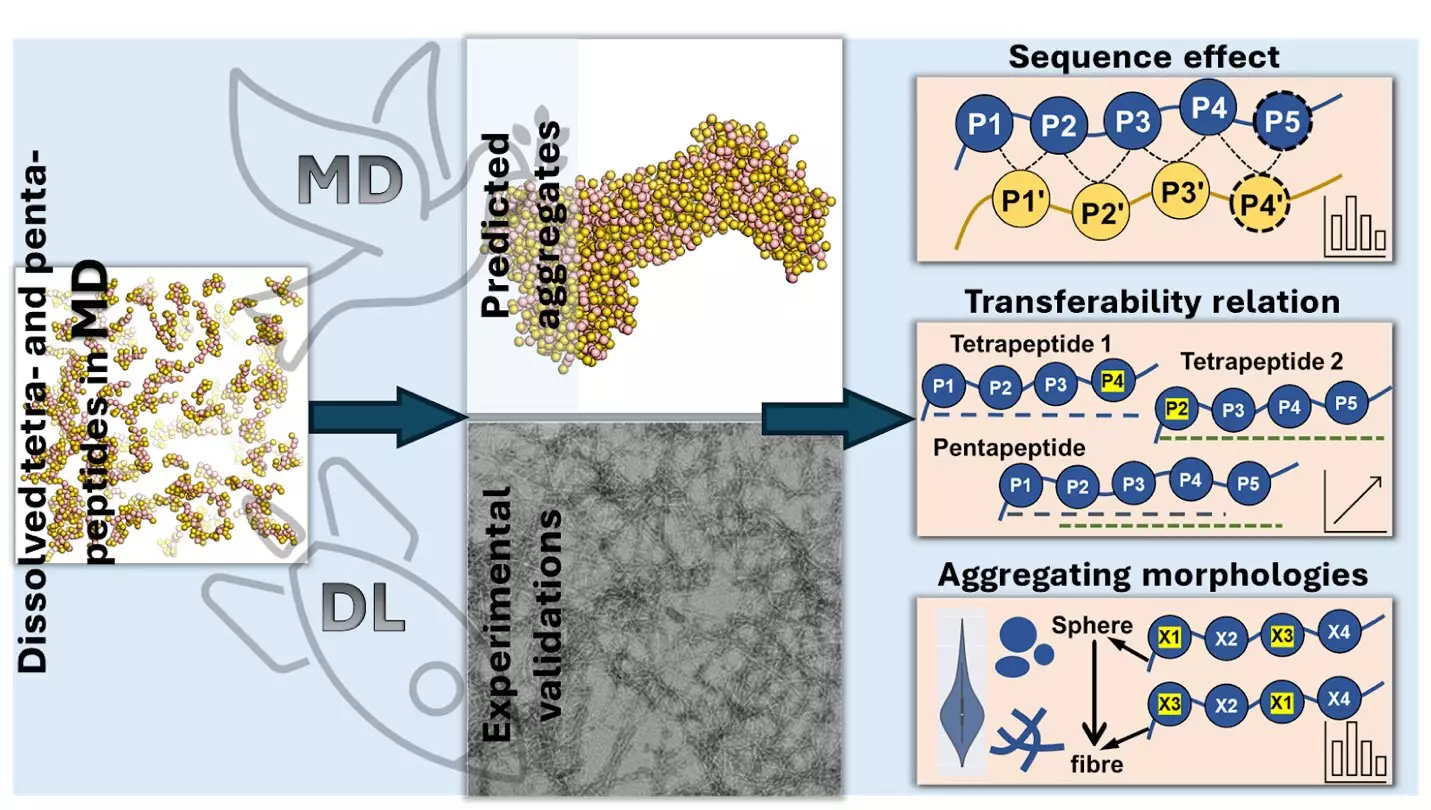
The research team employed molecular dynamics simulations and advanced AI techniques, including deep learning models like Transformer Regression Networks. Their goal was to predict the aggregation patterns of tetrapeptides and pentapeptides based on their amino acid sequences. Through analysis of 160,000 tetrapeptides and 3.2 million pentapeptides, the researchers identified the impact of different amino acids on the aggregation process.
Aromatic amino acids, particularly tryptophan, phenylalanine, and tyrosine, were found to significantly enhance aggregation, especially when located at the C-terminus of the peptide chain. These amino acids, with ring-shaped structures, attract each other through “π-π” interactions. In contrast, hydrophilic amino acids like aspartic acid and glutamic acid inhibit aggregation due to their strong interaction with water molecules.
The study revealed that altering the amino acid sequence has a direct impact on peptide aggregation. For instance, the addition of aromatic amino acids at the end of the peptide chain increases aggregation, while negatively charged amino acids at the beginning reduce aggregation. Additionally, the types and positions of amino acids influence the shapes in which peptides aggregate.
Dr. Wenbin Li, an assistant professor at Westlake University and corresponding author of the study, highlighted that understanding peptide aggregation can aid in predicting the behavior of longer peptides. This knowledge has significant applications in creating new materials, designing stable drugs, and addressing diseases associated with peptide aggregation, such as Alzheimer’s disease.
Dr. Jiaqi Wang, first author of the study and an assistant professor at Xi’an Jiaotong-Liverpool University (XJTLU), emphasized the potential of this research in improving biotechnology tools like semiconductors, biosensors, and diagnostics. The insights gained from studying peptide aggregation are poised to enhance biochemistry, materials science, and computational biology.
The study exemplifies the integration of artificial intelligence into scientific discovery, showcasing the synergy between traditional research methods and cutting-edge technologies. By combining molecular dynamics simulations with deep learning models, the researchers were able to make significant strides in understanding peptide aggregation.
Ultimately, the research conducted by the Chinese scientists paves the way for innovative applications in drug development, material design, and disease prevention. The intricate relationships between amino acids and peptide aggregation offer a glimpse into the complex world of protein interactions, opening doors to new possibilities in various scientific disciplines.

Leave a Reply