In recent years, the quest for sustainable solutions to combat climate change has intensified, leading researchers to explore the conversion of carbon dioxide (CO2) into valuable chemicals. A pivotal study published in *Nature Energy* has employed cutting-edge spectroscopic techniques and theoretical analysis to illuminate the complex pathways involved in transforming CO2 into high-demand substances like ethylene and ethanol. This research, spearheaded by Dr. Arno Bergmann and his team, not only enhances our understanding of CO2 electroreduction but also sets the stage for innovative practices in the chemical industry aimed at reducing the carbon footprint.
Electrochemical reduction of CO2 (CO2RR) serves as a revolutionary technology that harnesses renewable energy to convert greenhouse gases into commercially valuable products. Ethylene and ethanol emerge as focal points of this investigation due to their significance in producing biodegradable plastics and renewable fuels, respectively. Historically, the mechanistic biology underlying CO2RR remained a black box, which hindered the rational design of catalysts necessary for efficient production. This research breaks new ground by elucidating certain critical intermediate steps in the reduction process, which could lead to more effective catalytic designs.
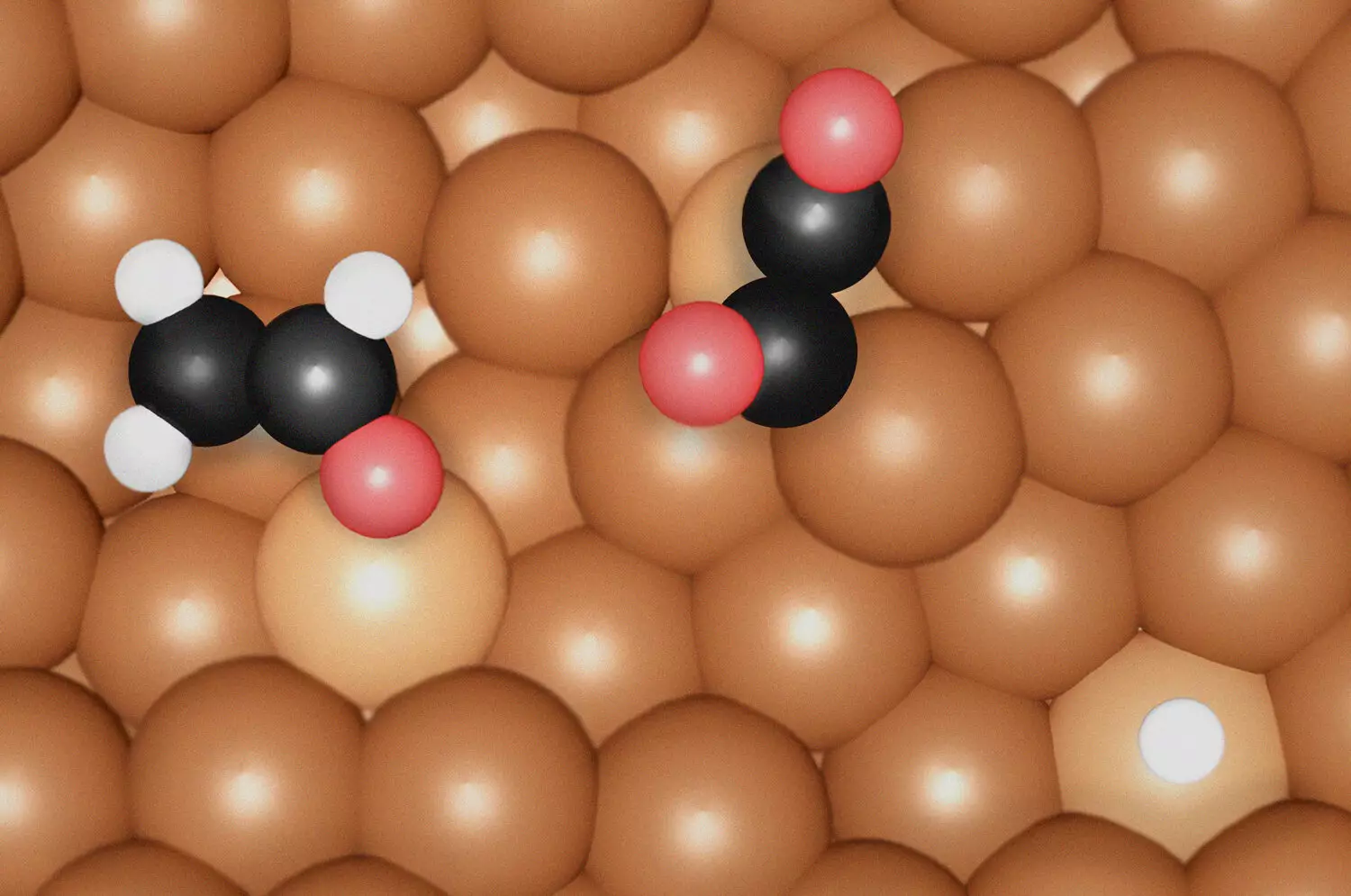
By employing in-situ surface-enhanced Raman spectroscopy (SERS) and density functional theory (DFT), the research team examined molecular dynamics on copper (Cu) electrocatalysts, providing invaluable data on how chemical intermediates influence the reaction pathway. One of their primary findings suggests that the formation of ethylene is associated with specific intermediates termed *OC-CO(H) dimers*, which evolve on undercoordinated Cu sites. In contrast, ethanol production relies on a uniquely compressed coordination environment of Cu sites, requiring the presence of the *OCHCH2* intermediate.
A particularly noteworthy insight from this research is the function of surface morphology in catalytic reactions. The undercoordinated Cu sites, marked by atomic-level irregularities that likely form under reaction conditions, are instrumental in enhancing CO binding strength—an essential phase in the reduction process. This discovery reveals a critical connection between the structural anomalies of catalysts and their improved performance, particularly in facilitating the formation of desired products.
The ability to tailor the catalytic environment for specific reactions signifies a breakthrough in catalyst design. By understanding how varying conditions affect the active site structure, researchers can progress toward developing catalysts that not only promote CO2 conversion but do so with optimal efficiency. This shift toward precise control of reaction pathways stands to revolutionize the industrial use of CO2.
The repercussions of these findings extend far beyond academic curiosity; they possess profound implications for the chemical industry. By utilizing insights into the specific intermediates and conditions that enable the selective production of ethylene and ethanol, chemists can devise more sustainable catalytic processes. This advances the overarching goal of reducing greenhouse gas emissions while producing high-value chemicals.
The collaboration between research groups, especially one in Spain contributing theoretical support, underscores the importance of interdisciplinary approaches in tackling complex scientific questions. Such collaborations facilitate a deeper understanding of the electrochemical CO2 reduction process and spark the development of revolutionary catalysts poised to reshape the future of chemical manufacturing.
The insights gleaned from this study represent a significant leap forward in our understanding of CO2 electroreduction. By uncovering the critical intermediates and active sites involved in producing ethylene and ethanol, the research paves the way for novel, more efficient catalytic approaches. This not only enhances scientific knowledge but also offers practical methodologies for mitigating CO2 emissions, thereby promoting sustainable practices within the chemical sector. As the world grapples with climate change, studies like this one illuminate pathways to turn pollution into productive resources, underscoring the potential for a circular economy rooted in sustainable chemistry practices.

Leave a Reply