At the forefront of energy innovation, the Fritz Haber Institute has unveiled groundbreaking insights into the realm of electrocatalysis, emphasizing the critical role of catalyst morphology—a factor long overshadowed by the focus on atomic-level active sites. The study, recently featured in *Nature Catalysis*, draws attention to the importance of a catalyst’s surface “roughness” and its profound influence on the selectivity of vital reactions, particularly those pivotal for sustainable energy, such as the transformation of CO₂ into usable fuels. This novel perspective not only reshapes our understanding of catalyst efficiency but challenges conventional wisdom by promoting a multifaceted approach to catalyst design.
Catalyst Morphology: The Unsung Hero of Electrocatalysis
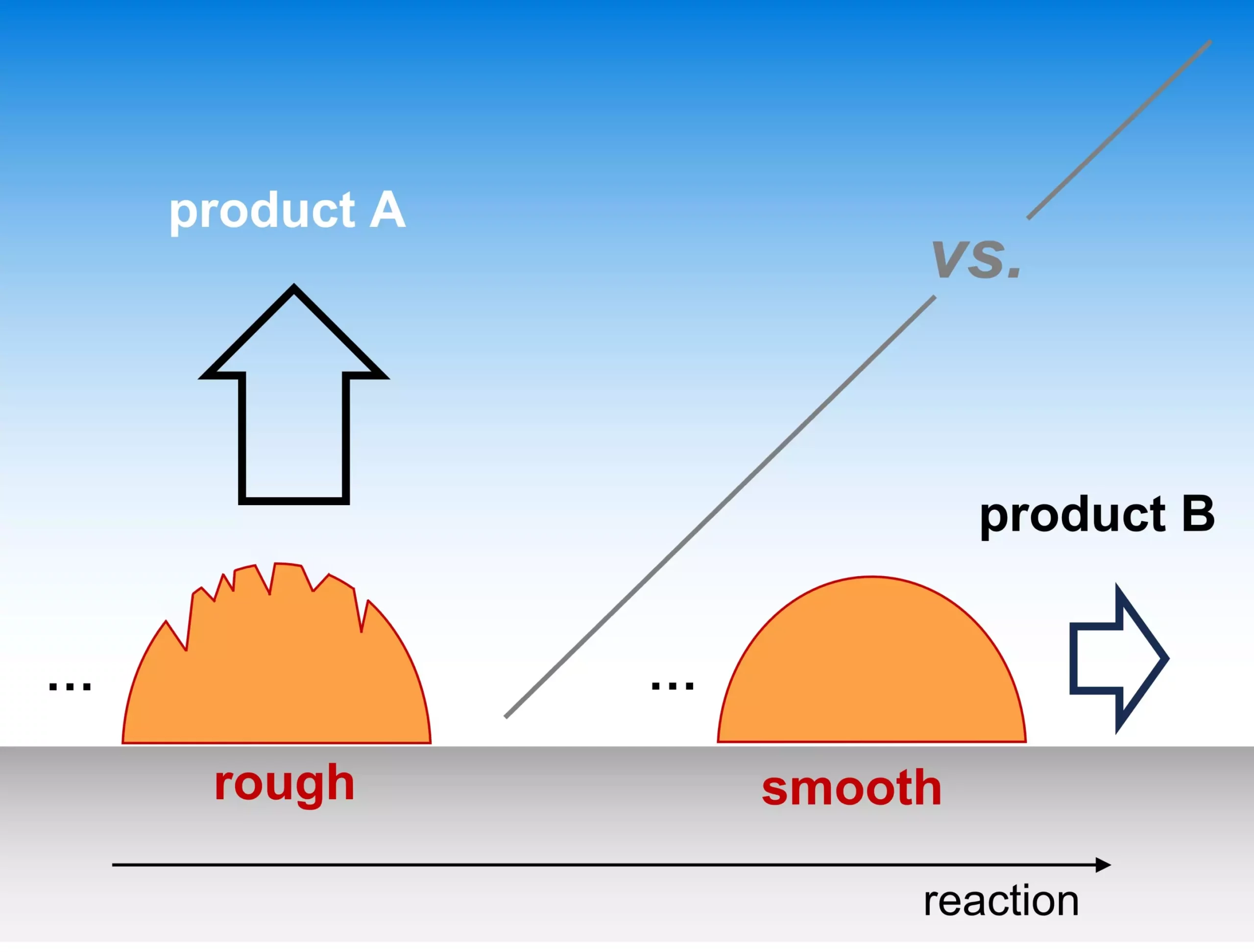
Catalysis, an essential component of the chemical industry, harnesses the potential to streamline processes ranging from plastic manufacturing to pharmaceutical advancements. Heterogeneous electrocatalysis stands out as a beacon of hope for sustainable energy solutions by enabling reactions under mild conditions through renewable electricity, ultimately aiming for a carbon-neutral future. Delving into catalyst morphology offers a new lens through which to assess efficiency. This analysis posits that a catalyst’s surface characteristics, specifically its roughness, directly dictate how effectively these catalysts can facilitate key reactions.
By shifting focus from the atomic attributes of active sites to the macroscopic properties of the catalyst’s surface, the research provides a comprehensive understanding of selectivity in electrocatalytic reactions—a subject shrouded in ambiguity prior to this study. The implications of this approach suggest that optimizing the physical structure of catalysts can lead to improved operational longevity and efficiency in energy production processes.
The Insights Behind Selectivity in Electrocatalytic Reactions
The investigation presents a compelling microscopic mechanism whereby reaction intermediates traverse the catalyst surface, sometimes escaping detection, complicating the evaluation of product output. By introducing a multi-scale kinetic model, the researchers articulate how the transport rate of reactants through the electrolyte significantly affects reaction outcomes. The model effectively quantifies the impact of active site density—essentially the roughness of the catalyst surface—on selectivity, bridging a gap in existing research.
Notably, the simplicity of this model belies its effectiveness, capable of mirroring trends observed in existing experimental literature. This reliability suggests the proposed mechanism might be universally applicable, providing a versatile framework for understanding catalytic behavior across various contexts. The robustness of these findings elevates them as critical insights for researchers and industries alike, promoting innovative pathways for electrocatalytic optimization.
A Future Shaped by Catalyst Innovation
The work spearheaded by the Fritz Haber Institute not only enhances our fundamental grasp of reaction mechanisms but also heralds a new direction for developing tailored catalysts. As the quest for sustainable energy intensifies, embracing the intricacies of catalyst morphology could very well be the key to unlocking more efficient, cost-effective processes. The emerging narrative around catalyst roughness is one of promise, urging both academic and industrial sectors to reconsider how catalysts are designed and implemented in sustainable technologies. As we navigate this transformative landscape, the synergy between morphology and performance will likely lead us towards a greener, more energy-efficient future.

Leave a Reply