In recent years, the nexus between lipids and various medical conditions has garnered increasing attention. A noteworthy advancement in this area comes from researchers at Würzburg and Berlin, who have unveiled a new molecule that significantly enhances our understanding of sphingomyelin metabolism. This discovery not only unveils new insights into the workings of sphingolipids but also paves the way for innovative therapeutic strategies in infection research. The findings were presented in the distinguished journal Nature Communications.
The Historic Journey: From Discovery to Disease
Back in the late 19th century, the German pathologist Ludwig Thudichum made a pivotal discovery when he isolated previously unknown fatty substances from brain tissue. He named these substances sphingolipids, an homage to the Sphinx of Greek mythology, recognizing the complex mysteries surrounding these molecules. Fast forward to today, it is evident that disturbances in sphingolipid metabolism are implicated in various diseases, particularly those affecting the brain. Conditions like Fabry’s disease and Gaucher’s disease serve as prime examples.
However, the implications of sphingolipids extend far beyond genetic disorders. Numerous infectious diseases, including Ebola, measles, and notably COVID-19, have been linked to sphingolipid metabolism. Bacterial pathogens such as Pseudomonas aeruginosa and Staphylococcus aureus also engage in metabolic manipulation during infections, shedding light on the role of sphingomyelin and its degradation in both viral and bacterial pathogenesis. Historically, the degradation of sphingomyelin, conducted by the enzyme sphingomyelinase, has emerged as a pivotal process in many of these infections, but visualizing this enzymatic activity has remained a challenging feat.
The team of researchers from Würzburg and Berlin embarked on the ambitious project of developing a novel sphingomyelin derivative capable of visualizing both the distribution of sphingomyelin and the activity of sphingomyelinase during infection processes. They are affiliated with the Research Training Group 2581, focused on investigating membrane proximal lipids and their signaling components in infections.
Professor Jürgen Seibel, from the Institute of Organic Chemistry at Julius-Maximilians-Universität Würzburg, asserts the complexity of creating trifunctional sphingomyelins that are metabolically compatible with natural sphingomyelin. These newly synthesized molecules possess three additional functional groups, expanding their utility in research settings. This trifunctional design allows for improved visualization techniques, enabling scientists to track metabolic processes in real-time.
The researchers demonstrated the application of these molecules by observing the activity of bacterial sphingomyelinase on human cell surfaces. They took this one step further, revealing sphingomyelin degradation within human cells during infections caused by intracellular Chlamydia bacteria. This particular pathogen is notorious for its associations with genital infections and potential cancer development in infected tissues.
Advanced Methods Yield Insightful Results
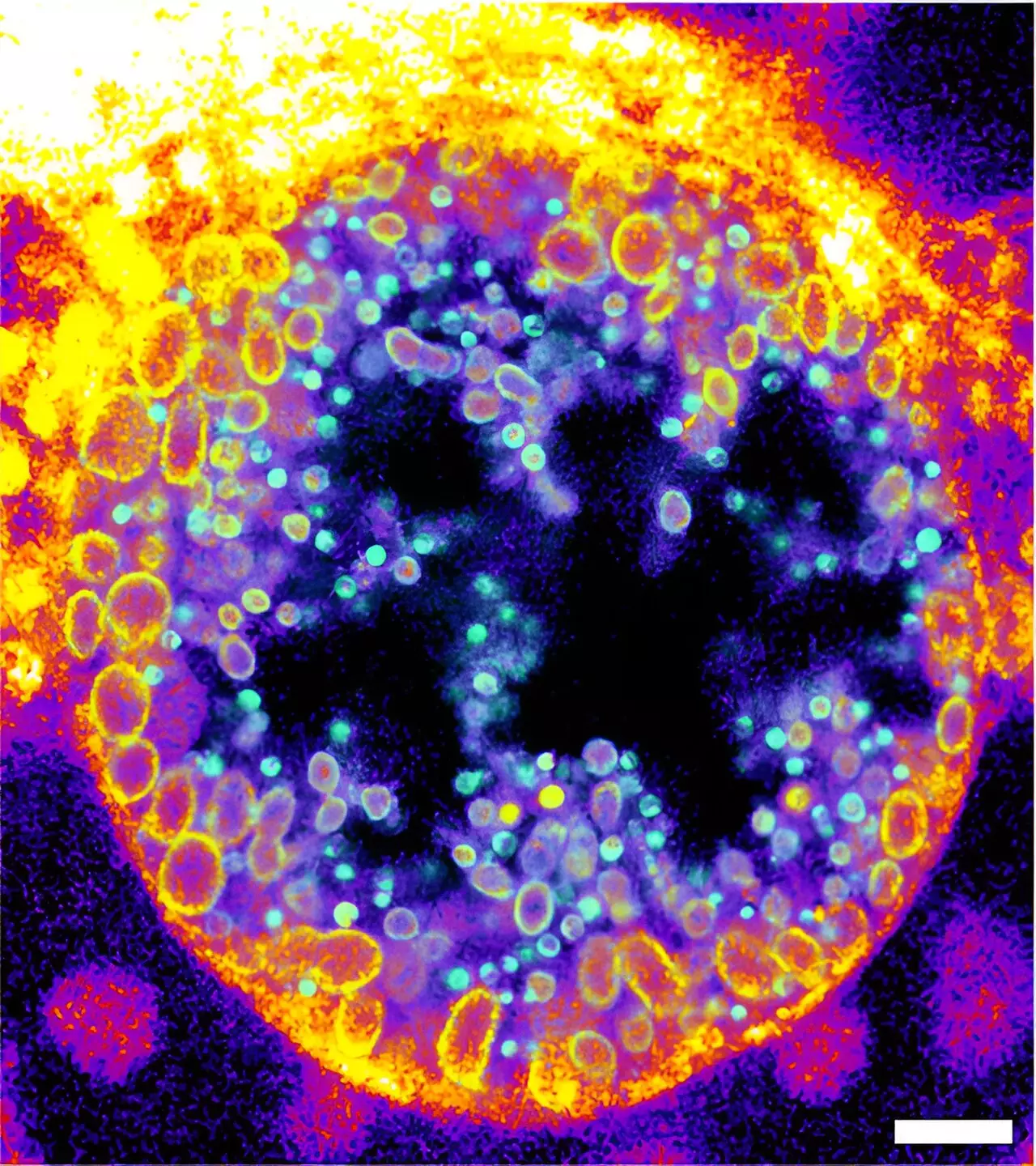
Chlamydia creates a replicative niche known as an inclusion within host cells, where it can hide from the immune system and proliferate. By utilizing cutting-edge techniques such as expansion microscopy and click-chemistry, the research team observed that during the maturation of Chlamydia from a non-infectious to an infectious state, there was a marked increase in the metabolized forms of their newly developed trifunctional sphingomyelins. This groundbreaking ability to visualize the infection process marks a significant leap forward in infection biology.
Professor Seibel emphasizes the immense potential of this new chemical tool, which not only provides a clearer understanding of sphingolipid metabolism but also opens up a realm of possibilities for developing targeted strategies against infections. This research underlines the importance of interdisciplinary collaboration, combining the expertise of chemists, physicists, and biologists, which has been vital in synthesizing these novel compounds.
The implications of this research are profound. By enabling researchers to visualize sphingomyelin metabolism in real-time, it may become possible to identify novel therapeutic targets within the sphingolipid pathway. As infectious diseases continue to pose a significant health threat globally, understanding their biochemical underpinnings is crucial for developing effective treatments.
The work of the Würzburg and Berlin researchers not only sheds light on the intricate behaviors of sphingomyelin in the context of infection but also underscores the potential that lies in rethinking therapeutic approaches targeting lipid metabolism. This landmark achievement illustrates the continuous evolution of scientific understanding and its pivotal role in addressing complex health challenges.

Leave a Reply