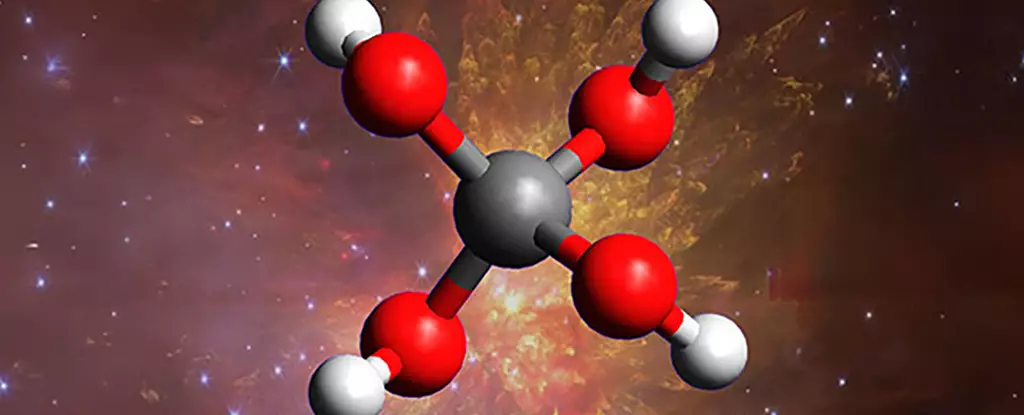
Science has entered a new era of discovery with the artificial creation of methanetetrol—a molecule long theorized but elusive until now. This revelation is not just a matter of confirming a predictive model; it fundamentally shifts how we understand the chemical complexity of space. For decades, scientists believed that the frigid, radiation-bathed environments of interstellar clouds might host extraordinary chemical reactions, but the actual molecules remained largely speculative. The production of methanetetrol in laboratory conditions provides tangible proof that the cosmos is capable of hosting such advanced and unstable molecules, defying previous assumptions about space chemistry being simple or limited.
This breakthrough speaks volumes about the surprising resilience of chemistry even in the universe’s most extreme conditions. The process involved carefully mimicking the “space ice” within a controlled laboratory setting—freezing carbon dioxide and water at ultra-low temperatures inside a vacuum chamber—then bombarding this simulated environment with high-energy radiation to replicate cosmic rays. Such an approach underscores how experimental innovation is vital to unearthing the universe’s hidden chemical repertoire. It’s not just about seeing what can exist in space anymore; it’s about expanding our understanding of what *can* exist beyond the limitations of Earth’s stable and chemically predictable environment.
Implications for Space Chemistry and the Search for Life
The formation of methanetetrol opens an entirely new chapter in the field of astrochemistry, highlighting a universe teeming with complex, reactive molecules previously thought impossible. This molecule, with its quartet of hydroxyl groups attached to a single carbon, exemplifies how extreme conditions can foster what appears to be highly unstable chemistry on Earth. The fact that it can be synthesized under lab conditions suggests that similar reactions might naturally occur in the cold, radiation-drenched depths of space.
What excites many scientists is the potential link between these findings and astrobiology. If molecules as complex and unstable as methanetetrol can form, what other “impossible” molecules are awaiting discovery in the cosmic shadows? These molecules could serve as chemical building blocks for more complex organic compounds, pushing the boundaries of how we consider the origins of life. Detecting and understanding such molecules might be key to decoding the steps that lead from simple interstellar dust and ice to the emergence of living organisms, possibly on planets or moons that orbit distant stars.
Moreover, this discovery encourages a reevaluation of existing models of space chemistry, which often rely heavily on stable, terrestrial-like reactions. The evidence suggests that the universe is a far more dynamic and unpredictably reactive environment than we had anticipated, rife with transient molecules that may leave fleeting but significant imprints on the cosmic landscape.
Challenges and Future Horizons in Detecting ‘Super Alcohols’
Despite the strides made in laboratory synthesis, the path to observing methanetetrol in its natural interstellar habitat remains fraught with challenges. Its extreme instability means it quickly disintegrates when exposed to light, especially the intense radiation emitted by stars and cosmic phenomena. This dissociative photoionization renders direct detection in space exceedingly difficult, confining most observations to indirect clues and laboratory analogs.
Yet, this obstacle does not diminish the importance of the breakthrough. Instead, it galvanizes the scientific community to develop more sensitive, precise detection methods—advanced telescopes, spectroscopy techniques, and predictive chemical models. Knowing what to look for, scientists are now better equipped to attempt the fleeting detection of methanetetrol or similar molecules in space. The recent discovery of related molecules like methanetriol underscores the notion that space chemistry is vast and largely unexplored—an untapped chemical frontier.
There’s also a compelling optimistic undertone here: as technology advances alongside our understanding, the possibility of uncovering complex molecules in space grows ever more likely. Just as paleontologists dig through layers of earth to find remnants of ancient life, astrochemists are carefully sifting through the cosmic medium, thrilled by every new clue that could rewrite what we consider possible in the universe. The discovery of methanetetrol is a potent reminder of how much is left to discover—molecules that could hold the keys to understanding not only the chemistry of space but perhaps the origins of life itself.

Leave a Reply