Recent advancements in single-atom catalysis have uncovered significant insights into the behavior of metal catalysts, particularly with respect to their efficiency and performance. A remarkable study led by Prof. Yan Wensheng from the University of Science and Technology of China (USTC) has put forth a groundbreaking exploration of the nuanced relationship between metal loading and catalytic activity in oxygen evolution reactions (OER). The findings from this research not only challenge pre-existing assumptions but also pave the way for innovative breakthroughs in catalyst design.
The “Volcano-Type” Phenomenon Explained
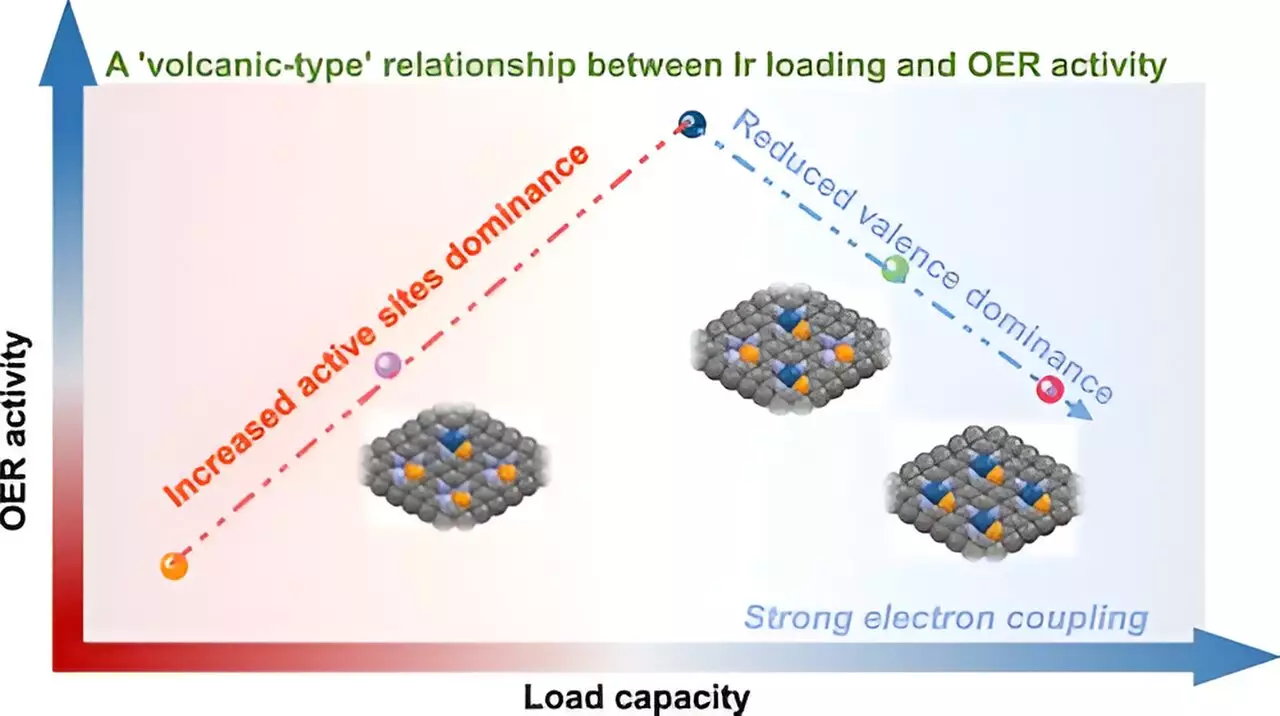
The classical understanding of catalyst behavior often suggested a straightforward linear increase in performance with higher metal loading. However, the USTC research team uncovered a more complex “volcano-type” relationship that emerges at elevated metal concentrations. This nuanced behavior indicates that while increasing metal loadings up to a certain point enhances catalytic performance due to a rise in active sites, beyond this threshold, it can hinder performance due to excessive metal interaction. This paradoxical behavior calls attention to the crucial balance required in catalyst formulation, one that has profound implications for industrial applications and sustainability.
Methodologies that Redefine Standards
Utilizing a straightforward P-anchoring strategy, the researchers successfully synthesized a series of iridium (Ir) single-atom catalysts with metal loadings ranging from 5% to a striking 21%. The importance of synchrotron radiation X-ray absorption spectroscopy (XAS) cannot be overstated in this endeavor, as it provided the clarity needed to understand the coordination between Ir and phosphorus (P). This interaction plays a pivotal role in maintaining the stability of Ir atoms during the catalyst’s performance at high loadings, effectively preventing unwanted aggregation—a common hurdle in catalyst manufacturing.
Implications for Future Research and Applications
The implications of this study are not limited to the immediate field of catalysis. The elucidation of the “volcano-type” relationship enriches the theoretical framework surrounding metal loading strategies and could potentially transform practices across various catalytic reactions. As industries seek to develop more efficient and cost-effective catalysts for energy conversion and environmental sustainability, understanding these intricate electronic interactions becomes increasingly indispensable.
Moreover, subsequent experiments utilizing X-ray photoelectron spectroscopy (XPS) and theoretical calculations allowed for greater insight into the degradation of catalytic efficiency at higher Ir loadings. Such multi-faceted research methodologies are essential for demystifying the electronic transformations that underpin catalytic behavior.
Charting the Course for Green Chemistry
In an era marked by an urgent need for sustainable solutions, this research signifies a critical leap toward optimizing catalysts that not only perform efficiently but are also economically viable. As single-atom catalysts garner interest in the realm of green chemistry and renewable energy technologies, these findings can serve as a beacon, guiding researchers and industries alike in their quest to innovate and optimize catalytic processes. The study’s revelations not only strengthen our understanding of catalytic behavior but highlight the vibrant potential that lies within the realm of single-atom catalysis. Such advancements can propel us toward significant environmental solutions, revolutionizing practices in energy production and usage.

Leave a Reply