In an era marked by rapid advancements in cancer research, two neutron experiments conducted at the Department of Energy’s Oak Ridge National Laboratory (ORNL) have unveiled critical insights into the enzyme serine hydroxymethyltransferase (SHMT). These experiments, utilizing advanced neutron techniques at the Spallation Neutron Source and the High Flux Isotope Reactor, have provided unprecedented clarity on the atomic-level chemistry involved in SHMT function. This discovery promises to aid the design of innovative drugs aimed at combatting aggressive cancer types, capitalizing on the enzyme’s central role in cell division.
Cancer exploits the body’s metabolic pathways, especially those involving enzymes like SHMT, to facilitate rapid cell proliferation. By hijacking these chemical reactions, cancer can turn a well-controlled process into one that accelerates uncontrolled growth. The challenge, therefore, lies in designing inhibitors that can effectively target and incapacitate SHMT early in the metabolic process, theoretically dismantling the cancerous growth mechanism.
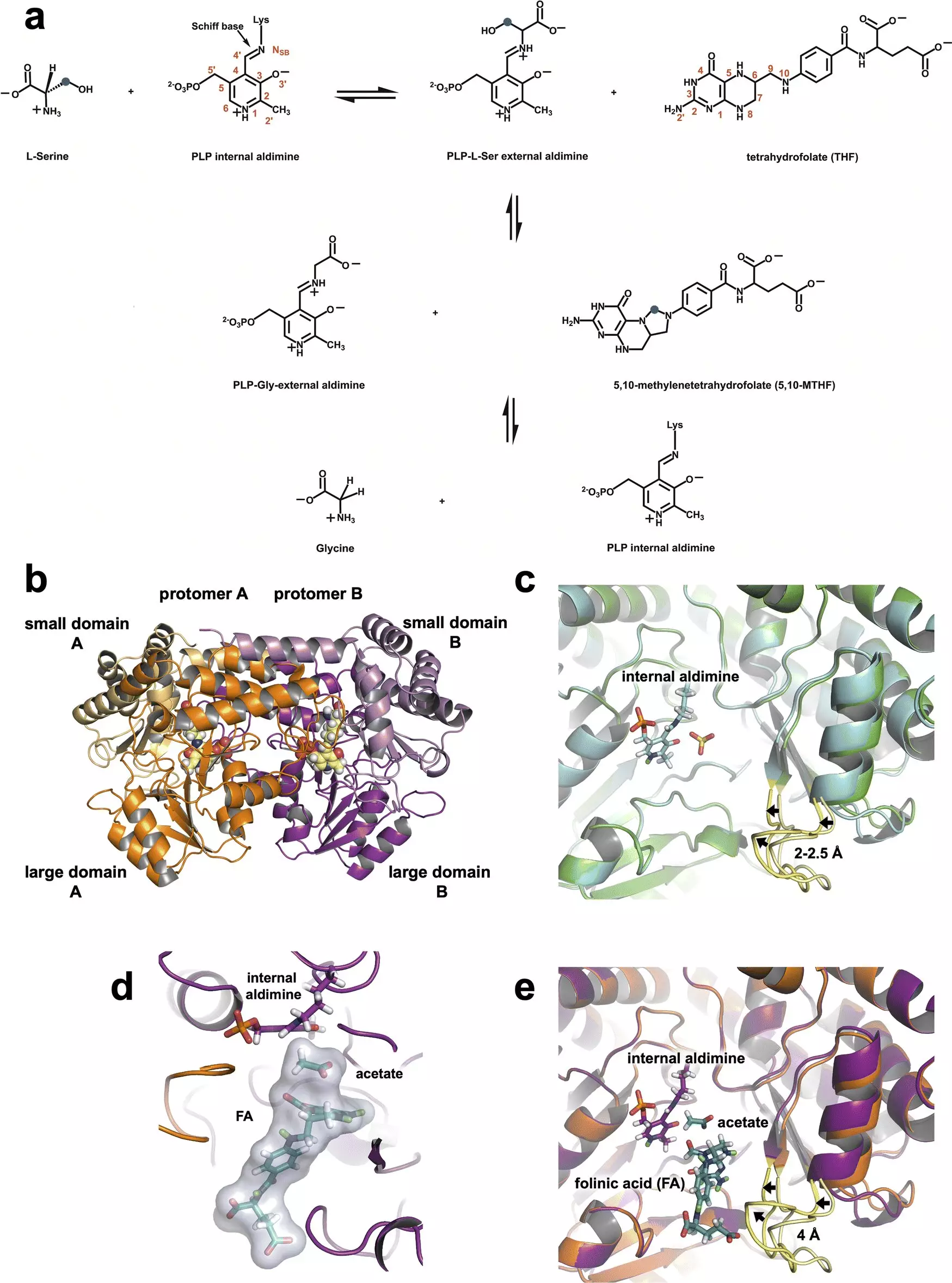
The intricacies of SHMT and its catalytic mechanisms have long been subjects of scientific debate. However, the recent research provides definitive observations concerning the enzyme’s operations. Key among the findings is the role of a single amino acid residue, glutamate, found to be pivotal in regulating SHMT’s chemical reactions. According to biochemist Victoria Drago, one of the study’s lead authors, the neutron data highlights that the glutamate retains a proton, enabling it to function as both an acid and a base—a dual role that is crucial for facilitating the enzyme’s reactions.
In the one-carbon metabolism pathway, SHMT operates within cell mitochondria, orchestrating the conversion of serine to glycine while transferring a carbon atom to tetrahydrofolate. This conversion generates essential building blocks for synthesizing nucleic acids, which are vital for all cellular functions, particularly during cell division. By establishing the precise mechanisms at play, researchers have laid the groundwork for developing targeted inhibitors to obstruct SHMT’s activity.
A significant breakthrough in the study of SHMT stems from the researchers’ innovative use of neutron diffraction alongside X-ray crystallography. This combination allowed them to visualize the structure and function of the enzyme at physiological temperatures, providing insights that have previously been elusive. Neutrons effectively illuminate light elements, particularly hydrogen, which constitute a significant portion of enzyme structures and influence their electrostatic environments.
Drago emphasized the importance of hydrogen in biological chemistry, stating, “Neutrons allow us to see hydrogen atoms, and hydrogen drives chemistry.” Through these advanced techniques, the team has elucidated the roles of various amino acids in the enzyme’s active sites, which are essential for its catalytic function.
The implications of this research stretch beyond mere academic curiosity; they represent a potential turning point in cancer therapeutics. SHMT, when overexpressed in cancer cell mitochondria, presents a logical target for drug design. The early intervention in the one-carbon metabolic pathway could significantly impede the progression of cancer. Unlike traditional treatments that require targeting our own body’s proteins, which often leads to undesirable side effects, an inhibitor designed for SHMT could provide a focused therapeutic approach with minimal collateral damage.
As highlighted by William Nelson, director of the Sidney Kimmel Comprehensive Cancer Center, the potential of integrating artificial intelligence with these advancements could someday revolutionize the cancer treatment landscape. “We’re moving closer to a point where we can predict a protein’s structure and synthesize a specific drug rapidly,” he remarked. While this future is not yet realized, the current findings are indispensable for guiding the next steps in inhibitor design.
Despite this promising progress, cancer treatment remains fraught with complexities. The adaptable nature of cancer cells can frequently allow them to recalibrate their metabolism in response to therapeutic pressure. Traditional regimens may falter as cancer evolves to exploit alternative pathways. Therefore, a multi-faceted approach, harnessing the latest insights from neutron studies, will likely be essential in developing an effective arsenal against cancer.
In summation, the combination of neutron diffraction and biochemical targeting of SHMT marks a significant step forward in our quest to understand and combat cancer. As researchers continue to investigate the atomic-level mechanisms of critical enzymes like SHMT, the path to more effective and less harmful cancer therapies may become clearer. Further research in this area holds promise not only for enhancing our understanding of cancer biology but also for improving patient outcomes through innovative drug development.

Leave a Reply