Cancer remains one of the most pressing health challenges globally, necessitating ongoing research into effective treatments. A crucial aspect of combating cancer lies in stopping the proliferation of cancer cells, which primarily relies on the intricate network of proteins that support their survival. Recent advancements in protein profiling have emerged as a promising avenue for drug development, offering a deeper understanding of potential therapeutic targets. This article delves into a pioneering approach from Scripps Research, highlighting the significance of their breakthrough findings in the realm of cancer treatment.
At the core of effective cancer treatment is the need to disrupt the interplay between cancer cells and their essential proteins. Understanding how these proteins function is critical, as they can often be the target of new drugs. Traditional methods of protein profiling have faced limitations; they frequently failed to identify all viable protein targets, which may have left significant opportunities for therapeutic intervention unaddressed. The recent study conducted by chemists at Scripps Research seeks to rectify these shortcomings by employing a dual methodology that enhances the sensitivity and specificity of protein targeting.
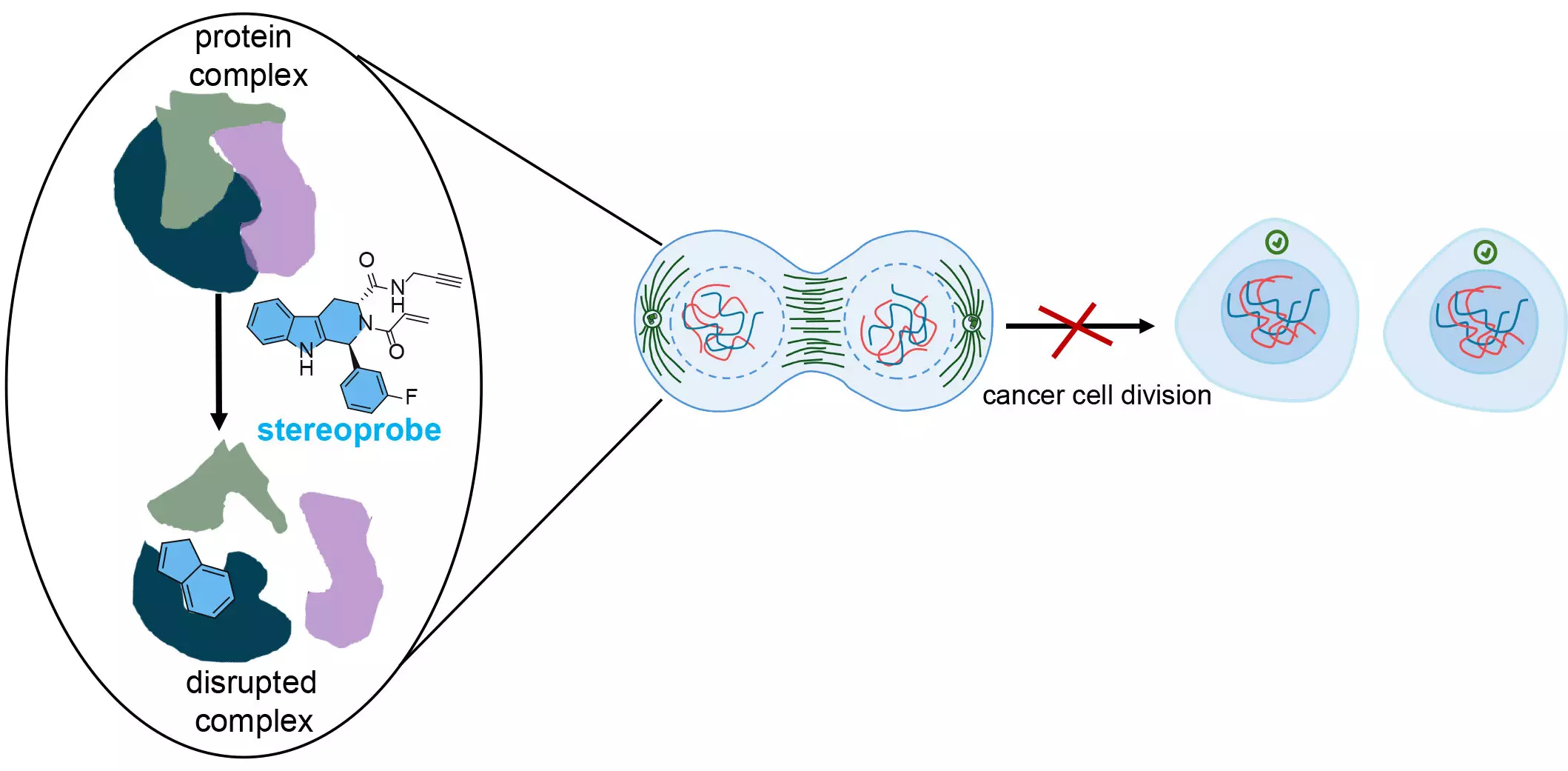
The research team has successfully mapped over 300 small molecule-reactive proteins, alongside their specific binding sites, by merging two advanced techniques of activity-based protein profiling (ABPP). Co-senior author Benjamin Cravatt, Ph.D., elucidates that one method provides a broad view of protein interactions, while the other delivers precise data on the location of these interactions. By integrating these techniques, the researchers enable a comprehensive understanding of how specific compounds interact with proteins, creating a roadmap for future cancer drugs.
The innovative approach involves the utilization of stereoprobes, which are designed to selectively and permanently bind to proteins. These sophisticated chemical compounds facilitate the examination of protein functionalities and help identify potential drug targets. The study emphasizes the thoughtful design of stereoprobes with unique chemical properties that differ from those typically prevalent in drug discovery, thereby enhancing the chances of uncovering novel biologically significant interactions.
Among the various proteins analyzed, a significant focus was placed on cysteine—an amino acid known for its ability to form important structural connections within proteins. This focus stems from cysteine’s high reactivity, making it an ideal target for therapeutic intervention. The prevalence of cysteine in cancer cells positions it as a prime candidate for drug binding, as agents that disrupt cysteine interactions can halt critical cellular processes. The research team’s stereoprobes were designed to irreversibly bind to cysteines, reflecting a strategic choice to exploit this amino acid’s unique properties.
The dual methodology employed by the researchers facilitated the identification of over 300 proteins that bonded with stereoprobes. However, to maximize the potential of this approach, a second technique pinpointed precisely where these interactions occurred on the proteins. This detail-oriented analysis allows for a better understanding of how specific probes inhibit protein functions, pivotal for developing targeted cancer therapies.
The implications of the findings from this research are substantial. By confirming that a dual approach offers more comprehensive insights into protein-stereoprobe reactivity, the study reveals the limitations of relying on a single profiling technique. A wealth of potentially viable protein targets could be overlooked if only one methodology is employed, emphasizing the importance of multifaceted approaches in cancer research.
Furthermore, this innovative research raises exciting prospects for future cancer therapies. By specifically targeting critical stages in the cancer cell cycle, it may be possible to significantly slow the growth of these malignant cells. This could lead to new treatments that not only hinder cancer proliferation but also enable the immune system to recognize and eliminate defects in these cells.
The Scripps Research team envisions extending the utility of their stereoprobe libraries beyond cancer, proposing investigations into a range of diseases linked to protein dysfunction. There is a wealth of other illnesses associated with aberrant protein activity, including various inflammatory disorders, which could benefit from similar profiling techniques. The researchers point out that many proteins implicated in these conditions remain underexplored due to a lack of appropriate stereoprobes, signaling an important area for future research.
The dual-method protein profiling approach unveiled by Scripps Research stands to revolutionize the landscape of cancer therapy by improving the identification and targeting of critical proteins involved in tumor growth. Understanding the sophisticated interactions between proteins and therapeutic agents could pave the way for more precise and effective treatments, ultimately advancing the goals of personalized medicine in oncology and beyond.

Leave a Reply