Polymers, often described analogously to trains made up of individual cars, are vital compounds that play an essential role in various industries. Just as trains rely on the secure attachment between cars, polymers rely on strong chemical bonds between monomers—small repeating units that form the backbone of the polymer structure. The properties and functionalities of these polymers are inherently tied to the nature of the monomers utilized. Therefore, the quest for developing diverse and unique monomers is crucial for expanding the applications of polymers in fields such as biomedical engineering, energy storage, and microelectronics.
A cohort of researchers at Scripps Research has made significant strides in this area, unveiling a groundbreaking method to synthesize monomers with novel properties through a specialized reaction that employs nickel as a catalyst. This revolutionary approach, published in *Nature Synthesis* on August 8, 2024, opens up a plethora of possibilities for crafting polymers characterized by remarkable structural and functional diversity.
Lead author Dr. Keary Engle, a noted professor in the Department of Chemistry at Scripps Research, emphasizes the importance of this study in unlocking pathways to previously inaccessible materials. By utilizing earth-abundant metals like nickel, the research aligns with current trends in sustainable chemistry, showcasing an eco-friendly alternative to traditional synthesis methods that often rely on rarer and more toxic metals.
The Collaborative Synergy of Research Institutions
This study represents a collaboration between the Engle lab and polymer researchers from the Georgia Institute of Technology and the University of Pittsburgh. Their collective ambition was to determine if the innovative strategies employed in small molecule synthesis could be scaled to synthesize polymers with unique properties. Postdoctoral researcher Dr. Anne Ravn reflects on the relevance of altering the chemistry of building blocks to yield diverse final products: “The properties of polymers are very much dependent on the backbone chemistry,” she explains. Thus, the research aims to apply new techniques to enhance the functionality of macromolecules, driving advancement in numerous applications.
The extensive research team consists of early-career scientists, including graduate students and postdoctoral fellows, who contributed unique insights and skills to the project. This collective effort serves as an example of how interdisciplinary collaboration can lead to impactful scientific breakthroughs.
Nickel-Catalyzed Reaction: The Heart of Innovation
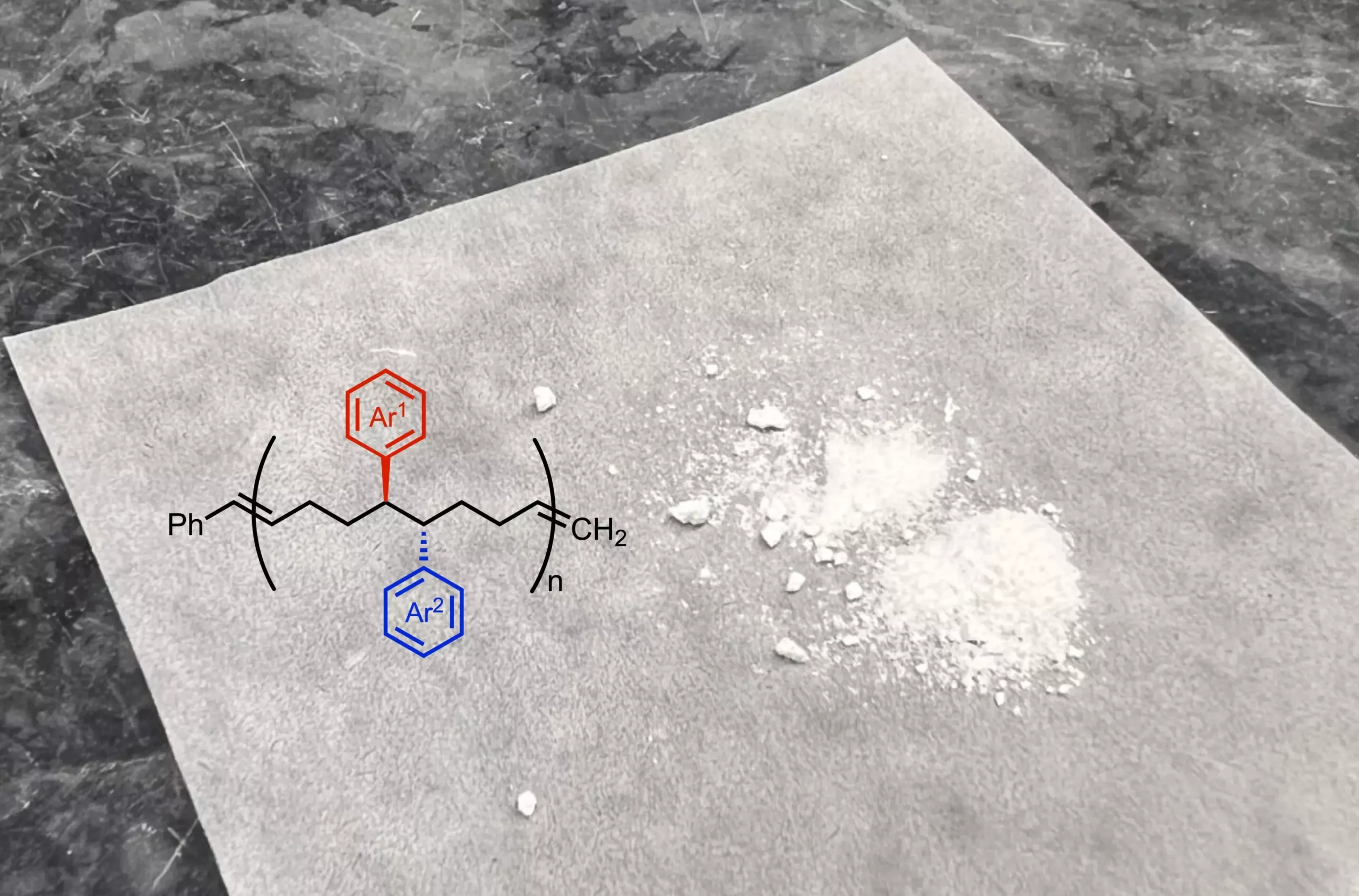
At the core of this research is the novel chemical reaction that modifies a precursor molecule through a nickel-catalyzed process. This reaction enables the addition of two functional groups to the initial compound, imparting distinctive properties that can alter the flexibility, elasticity, and solubility of the resulting polymer. Unlike conventional polymerization methods, which typically incorporate minimal variations in functional groups along the polymer chain, the new strategy allows for functional groups to be placed in closer proximity within the polymer. This characteristic promises to create materials with enhanced and diverse properties.
“Traditionally, most commercial polymers maintain a fairly uniform structure with identical functional groups,” Dr. Ravn notes. “Our approach significantly increases the spatial density of functional groups, leading to innovative material functionalities.”
As the research team looks to the future, they are enthusiastic about further exploring the potential of their method. The ability to substitute various functional groups onto the monomers presents unparalleled opportunities for customization. Dr. Ravn articulates the vision for expanding this method: “We aim to introduce a range of functional groups and evaluate how these substitutions influence material characteristics. Our goal is to provide chemists with an expanded toolkit for polymer design.”
Moreover, the research also emphasizes the importance of sustainability in polymer production. The utilization of nickel, an earth-abundant metal, coupled with the ongoing exploration of methods to degrade polymers back to their original building blocks signifies a commitment to environmentally responsible practices in the chemical industry. Emphasizing the necessity for reusability, Dr. Ravn envisions a future where polymer materials are not only crafted for performance but also designed for cyclical life cycles.
The findings from the Scripps Research team signify a monumental leap in polymer chemistry, providing a foundational framework for the production of customized and eco-friendly materials. The innovative use of nickel catalysis and a focus on functional group variation heralds a new era in the development of versatile and sustainably sourced polymers, addressing global challenges across multiple sectors. As researchers continue to refine these techniques, the implications for advanced material applications—spanning healthcare to technology—will undoubtedly be profound, promising a brighter future informed by chemical ingenuity.

Leave a Reply