As the world grapples with the dire consequences of climate change, the search for clean and sustainable energy sources has intensified. Among these, hydrogen gas emerges as a leading candidate, primarily due to its remarkable energy density and the absence of carbon emissions during combustion. Despite being the most prevalent element in the universe, hydrogen is rarely found in its pure form. It typically exists as part of countless chemical compounds, necessitating advanced methods for extraction and utilization. Among various hydrogen carriers, ammonia has caught the attention of researchers and industry alike: its high hydrogen content (17.6% of its mass), widespread availability, and efficient transportation characteristics position it as a promising player in the hydrogen economy.
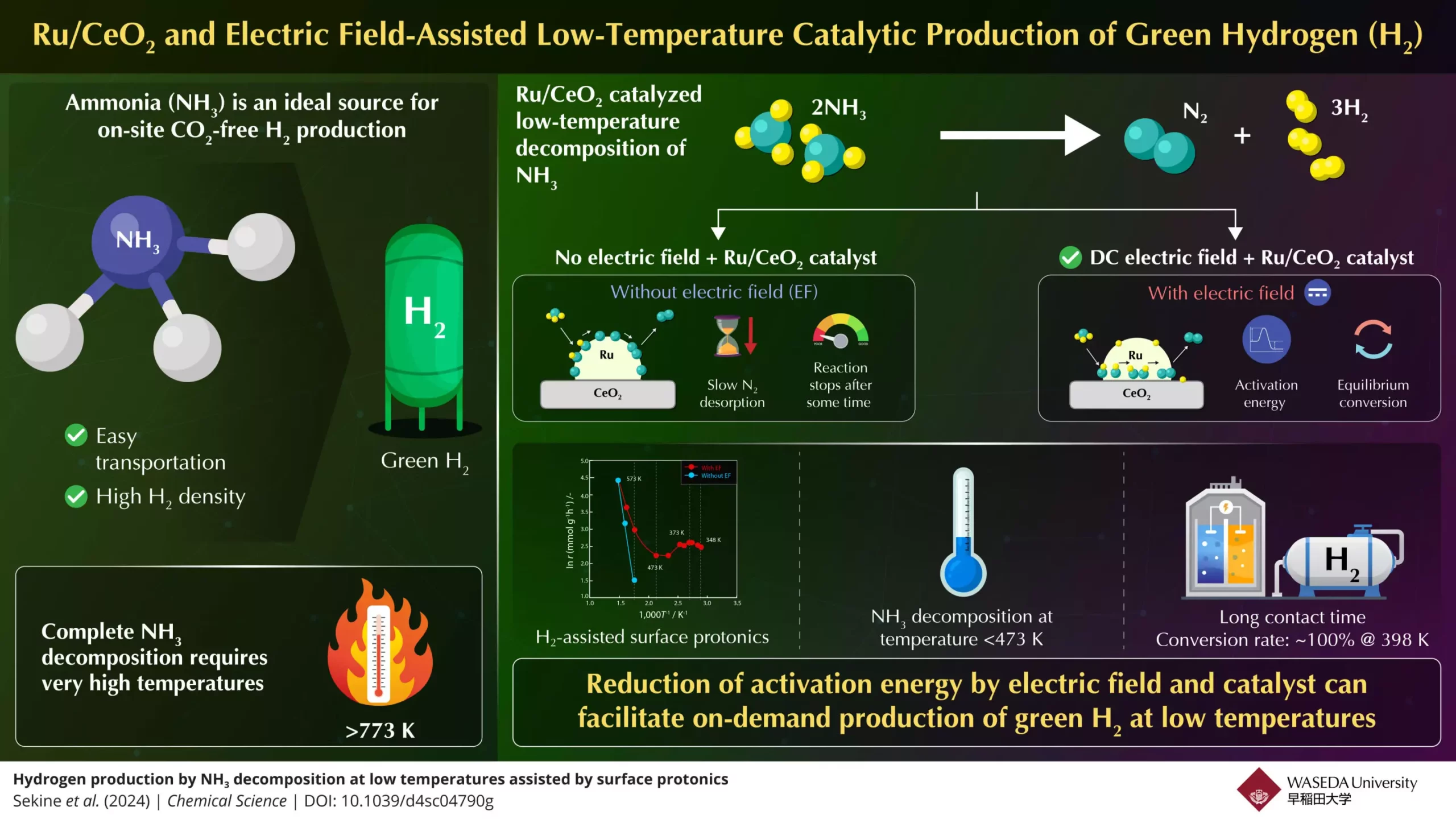
Despite its potential, the journey of ammonia from a carrier to an on-demand source of hydrogen faces significant hurdles. A substantial challenge arises from the high temperatures required for ammonia decomposition, generally exceeding 773K. Such extreme conditions not only complicate the process but also limit the widespread application of ammonia in fuel cells and internal combustion engines, where lower temperatures are often essential. Researchers are therefore under mounting pressure to innovate new methods that allow for efficient ammonia-to-hydrogen conversion under more feasible thermal conditions.
Recent advancements reported by a team at Waseda University, led by Professor Yasushi Sekine in collaboration with Yanmar Holdings, have illuminated a possible pathway to address these challenges. The team has pioneered an innovative process that leverages an electric field to stimulate ammonia decomposition at notably lower temperatures. Their setup, using a Ru/CeO2 catalyst, demonstrated high rates of ammonia-to-hydrogen conversion, marking a significant breakthrough in the field. This methodology offers a transformative approach, suggesting the viability of ammonia as a clean hydrogen source without necessitating extreme thermal conditions.
The core of the team’s findings lies in their examination of conventional thermal catalytic systems. Through their research, they identified that the desorption of nitrogen on active metal (Ru) was the limiting factor at low temperatures, while the dissociation of N–H bonds became the bottleneck at higher temperatures. By incorporating electric field-assisted techniques, they effectively enhanced proton conduction on the catalyst’s surface, thereby reducing the activation energy required for the ammonia decomposition process. This advancement not only facilitates a lower operational temperature—successfully achieving complete conversion rates below 473 K—but also allows for reaction efficiency that surpasses traditional equilibrium limitations.
The results yielded by Sekine’s team are particularly striking. With optimal contact time between ammonia feed and catalyst, they achieved a remarkable 100% conversion rate at temperatures as low as 398 K—an unprecedented accomplishment in the context of ammonia decomposition. Their findings underscore the significance of surface protonics, where the electric field assists proton transfer on the catalyst surface, thereby minimizing activation energy barriers.
Comparative observations revealed that in the absence of an electric field, the nitrogen desorption process significantly slowed, ultimately halting the decomposition reaction. This stark contrast further emphasizes the contribution of electric fields in enhancing ammonia conversion rates through robust experimental support and density functional theory calculations.
As the implications of this research sink in, they herald a new era for hydrogen production, indicating that ammonia can be leveraged as an efficient source of green hydrogen at low temperatures. This breakthrough not only paves the way for the practical implementation of ammonia in the burgeoning hydrogen economy but also sets the stage for widespread adoption of alternative fuel technologies that prioritize CO2-free energy production.
In essence, the innovative strategies introduced by Sekine and his collaborators mark a substantial step forward in hydrogen generation. By simplifying the process of obtaining hydrogen from ammonia, these developments could accelerate the transition to clean energy sources and ultimately contribute to a sustainable future free of carbon emissions. “We believe that our proposed method can accelerate the widespread adoption of clean alternative fuels by making the on-demand synthesis of CO2-free hydrogen easier than ever,” Sekine aptly notes, leaving us to ponder the vast potential that lies ahead within this disruptive technology.

Leave a Reply