Noble gases have long been characterized by their chemical inertness and stable electron configurations, making them seem largely unreactive under standard conditions. However, groundbreaking work conducted over six decades ago by Neil Bartlett challenged this assumption. By successfully synthesizing xenon hexafluoroplatinate (XePtF6), Bartlett not only introduced the first noble gas compound but also opened the door to the exploration of various other noble gas derivatives. Despite these advancements, the inherent difficulty in growing sufficiently large crystals containing noble gases has made detailed analysis of their structures particularly challenging. Recent efforts by researchers have begun to address these long-standing obstacles, revealing new insights into noble gas compounds.
Challenges in Crystallography
The fascination with noble gases is often hindered by their extreme sensitivity to environmental conditions, especially moisture. This chemical reactivity poses significant challenges in crystallography, as changes in humidity can lead to the degradation of noble gas crystals. As a result, many of these compounds have proven elusive in terms of structural characterization using traditional single-crystal X-ray diffraction techniques. Researchers often require intricate methods and specialized equipment to handle these sensitive materials, further complicating the quest for a comprehensive understanding of their properties.
In light of these challenges, recent developments in electron diffraction technologies have emerged as promising solutions for probing the structure of air-sensitive compounds, including xenon-based materials. Notably, a team of researchers, led by Lukáš Palatinus and Matic Lozinšek, recently applied 3D electron diffraction to investigate nanoscale crystallites of xenon compounds. This innovative method offers the potential to analyze crystalline structures without the size limitations that often accompany single-crystal X-ray diffraction, allowing researchers to explore new avenues in noble gas chemistry.
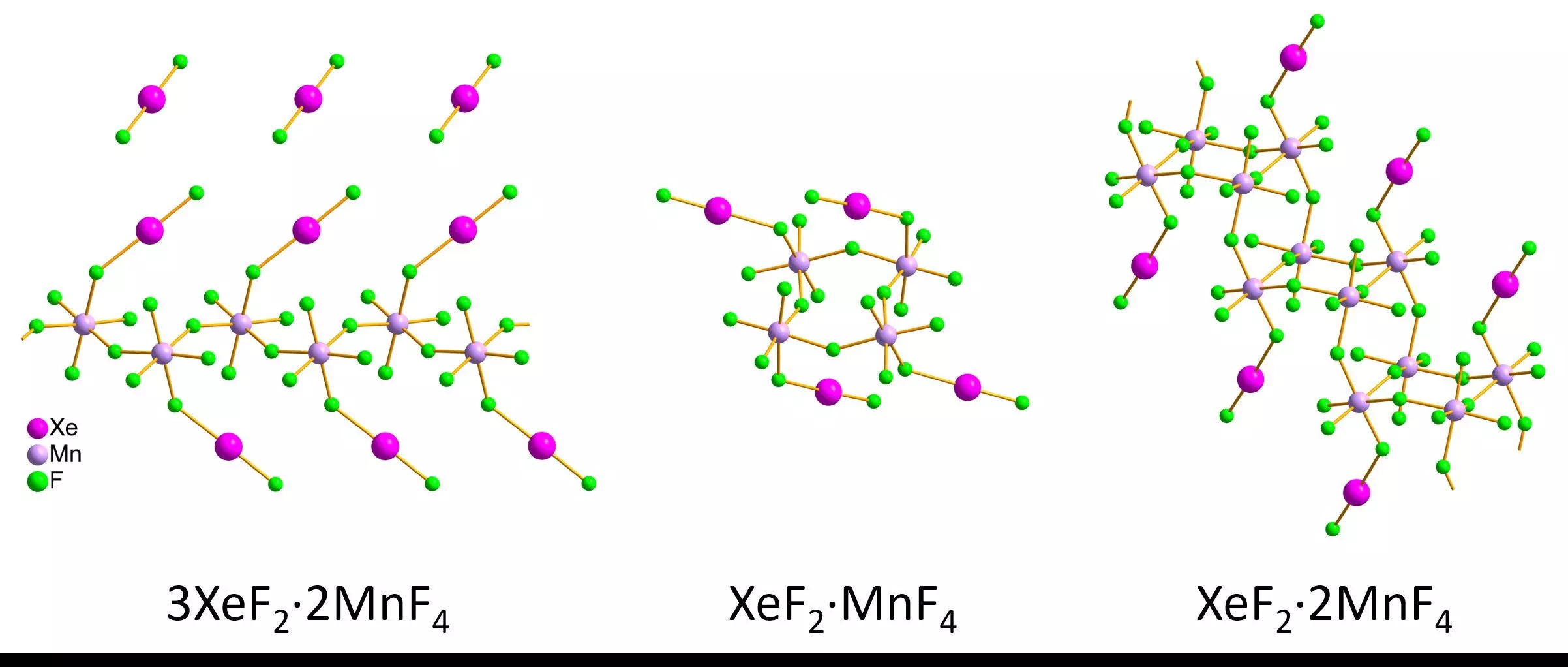
The researchers focused specifically on xenon difluoride-manganese tetrafluoride compounds, synthesizing various crystallites in both red and pink forms. They meticulously preserved the samples’ stability before inspecting them under a transmission electron microscope. The results yielded vital data regarding bonding characteristics, revealing the lengths and angles present in the xenon-fluoride and manganese-fluoride bonds. Comparison between the structures analyzed through 3D electron diffraction and traditional X-ray methods showed congruence in the overall geometry, despite minor discrepancies.
Implications for Future Research
The success of this study highlights the potential of leveraging 3D electron diffraction techniques to further unlock the mysteries surrounding noble gas compounds. With the ability to probe smaller and more fragile crystals, researchers now have the tools to investigate previously inaccessible structures, including the original xenon compound synthesized by Bartlett. The implications extend beyond noble gases: this technique could enhance understanding and characterization of various air-sensitive materials, proving transformative for the field of chemistry and paving the way for new discoveries in material science.
While noble gases have often been seen as chemically inert, the ongoing advancements in characterization techniques challenge this notion, revealing the diverse and complex nature of these elements and their compounds. The future appears bright for noble gas chemistry, as researchers strive to unlock the secrets of these fascinating materials.

Leave a Reply