The modern world is grappling with an escalating climate crisis that not only threatens the environment but also imperils human health on a cellular level. Carbon dioxide (CO2), a well-known greenhouse gas, has become a focal point of environmental discussions; however, its impact on human biology was previously less understood. Recent research by Ohara Augusto from the University of São Paulo sheds light on how elevated CO2 levels can lead to significant alterations in biochemical processes within our cells—enter peroxymonocarbonate, a compound that presents a fascinating yet alarming new dimension to our understanding of cellular health.
The research illuminates a crucial relationship: CO2 interacts with hydrogen peroxide (H2O2) in physiological environments, leading to the formation of peroxymonocarbonate. While hydrogen peroxide is traditionally recognized as a metabolite involved in oxidative stress, peroxymonocarbonate’s emergence as a metabolic intermediate suggests there are profound implications for both cellular signaling and dysfunction. As cities contend with rising CO2 levels, it is essential to explore how these changes can affect human health beyond just climate concerns.
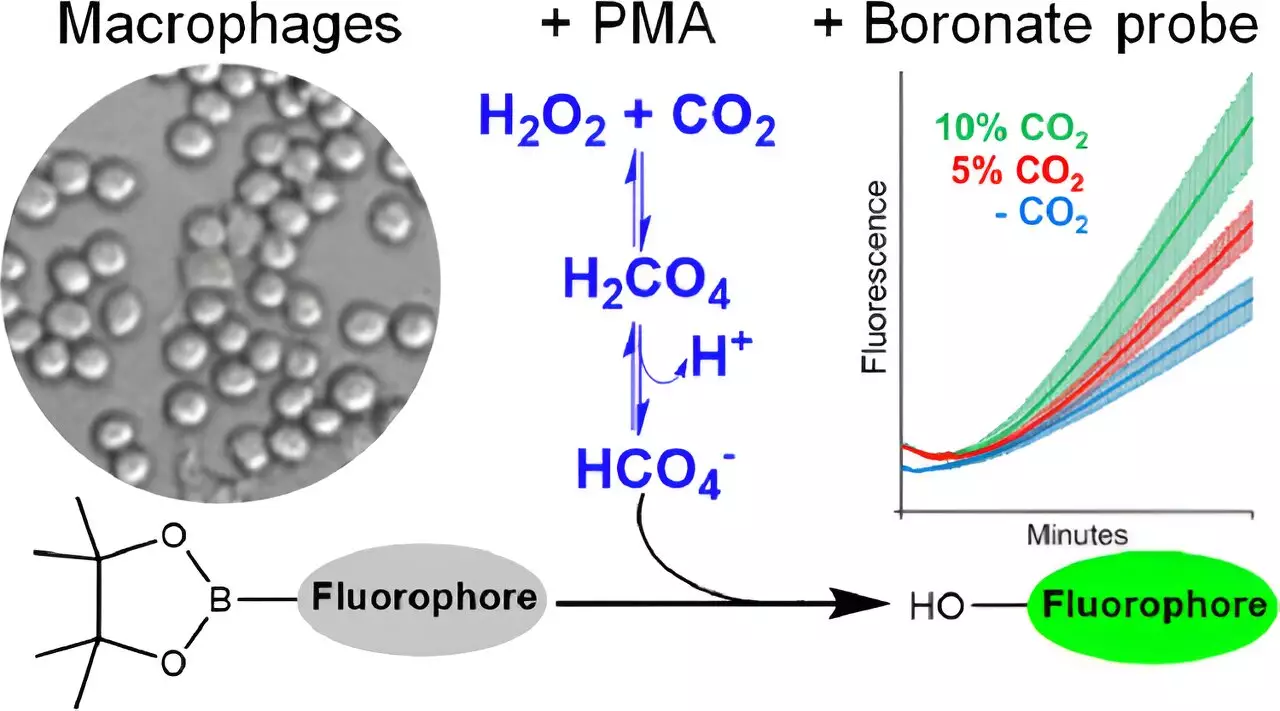
Augusto and his team made notable progress in detecting peroxymonocarbonate in cells by developing innovative fluorescent molecular probes. This is the first instance of detecting peroxymonocarbonate within a biological context, enabling the scientific community to confront the previously under-explored implications of CO2 exposure. Until now, the presence of this oxidant in living tissues was considered impossible due to the low concentration of its precursors and the slow rates of formation.
This research, published in the journal Chemical Research in Toxicology, not only introduces detection methods but also promotes dialogue around an overlooked area in the study of redox biology. Through their experiments, the researchers managed to narrow down the cellular conditions under which peroxymonocarbonate is produced, emphasizing the necessity to expand our understanding of CO2’s biological effects.
The methodological framework for studying peroxymonocarbonate hinged on analyzing fluorescence from boronate probes amidst varying levels of CO2 and hydrogen peroxide. By activating macrophages—an essential component of the immune system—the researchers could induce the production of hydrogen peroxide and observe the subsequent formation of peroxymonocarbonate without generating other harmful oxidants like peroxynitrite or hypochlorous acid.
By measuring the reactions in a controlled setting, researchers can conclude that the oxidative responses seen in cellular environments may stem largely from peroxymonocarbonate rather than just hydrogen peroxide as previously believed. This finding has potential ramifications for understanding oxidative stress and related diseases, as it opens up new avenues for therapeutic exploration centered around modulating these oxidants.
Redox signaling is a complex mechanism in which cells adapt to stress by signaling pathways that often modulate gene expression. When subjected to mild oxidative stress, cells can exhibit a protective response, producing antioxidant enzymes that mitigate potential damage. Notably, peroxymonocarbonate accelerates the oxidation of thiol proteins faster than hydrogen peroxide, amplifying its significance in redox processes.
But therein lies a complicating factor; while peroxymonocarbonate can serve a protective role, excessive production leads to irreversible oxidative damage. As CO2 levels rise, the potential for peroxymonocarbonate formation may increase, thus heightening the risk of cellular dysfunction and possible illness.
The implications of Augusto’s research extend far beyond mere laboratory findings. As CO2 concentrations in urban settings approach levels that can instigate physiological problems, understanding how compounds like peroxymonocarbonate function becomes crucial. CO2 not only affects the oxidative balance of cells but also plays a role in gene expression tied to inflammation and immune responses.
The concept of protein carbamylation and nitration broadens the discussion of CO2’s role in post-translational modifications, hinting at how chronic exposure to elevated CO2 can affect long-term protein function and cellular behavior.
The increasing levels of carbon dioxide in our environment may represent a significant threat to our cellular integrity and health, with peroxymonocarbonate at the center of this emerging narrative. Though further investigation is essential, what remains clear is that the consequences of climate change reach deeper than we previously understood; they could dictate the very processes of life within us.

Leave a Reply