In an era where medical science and technology converge, the University of Virginia’s School of Engineering and Applied Science stands on the brink of a remarkable advancement. A collaborative effort led by Professor Liheng Cai and Ph.D. candidate Jinchang Zhu has produced what may be the foundational elements for on-demand human-compatible organ production. Their recent publication in *Nature Communications* accentuates not only a significant leap in bioprinting capabilities but also the potential for revolutionary changes in healthcare assistance through organ regeneration and disease modeling.
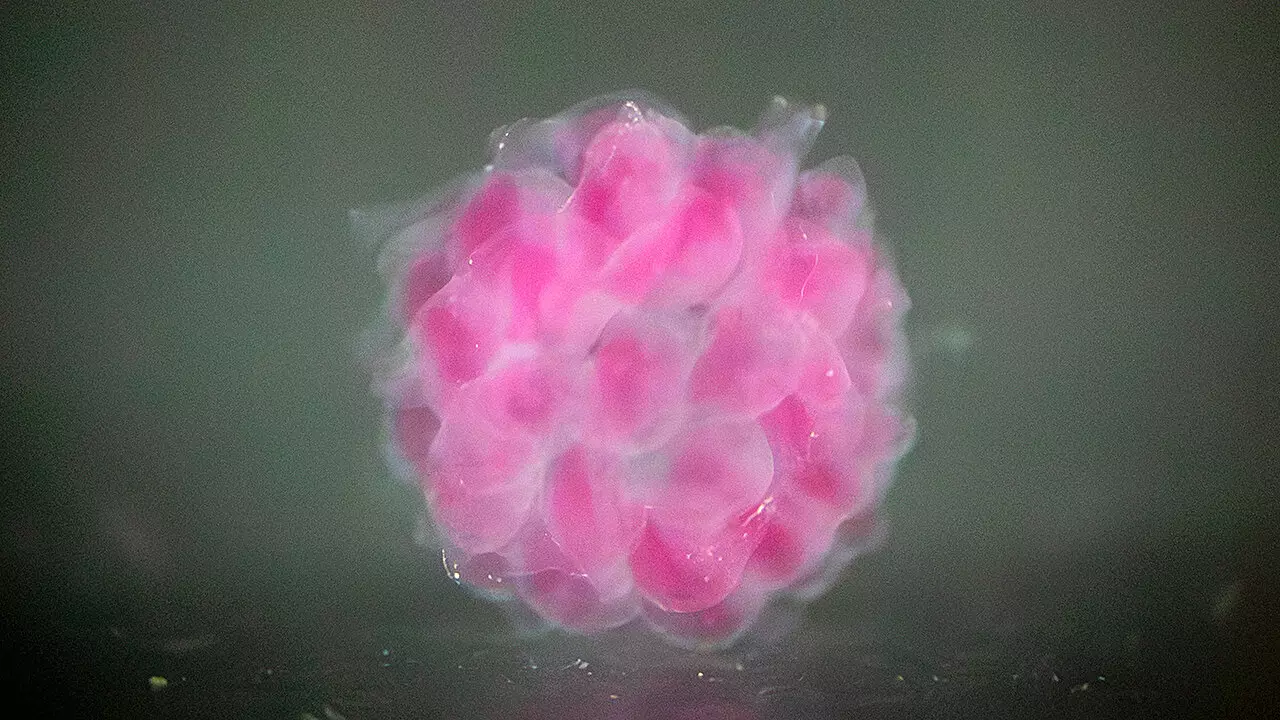
The research team has pioneered a unique bioprinting method known as Digital Assembly of Spherical Particles (DASP), which meticulously constructs 3D structures that closely replicate various human tissues. The core of this technique involves using controlled mechanical properties of biomaterials, which Zhu highlights as a substantial deviation from current bioprinting practices. Compared to existing technologies, which often fall short in mimicking the dynamic sensations and functions of human tissues, DASP exhibits unprecedented precision and versatility.
A Deep Dive into DASP Technology
The DASP methodology introduces a novel approach by utilizing hydrogel particles suspended in a supportive matrix, both of which are primarily water-based. This innovative assembly mimics the pixel structure of digital images but in a three-dimensional form—complex structures defined not by two coordinates but by the interactive properties of materials within human tissues. The researchers have ingeniously crafted these hydrogel particles to not only safely encapsulate human cells but to greatly enhance their viability and functionality in a controlled environment.
The significance of their bioprinting technology doesn’t merely lie in its innovative structure but also in its capability to mimic critical mechanical properties of human tissues. Thanks to their double network hydrogels, which feature intertwining molecular networks, the printed constructs boast robust mechanical strength while maintaining much-needed tunability. This balance can potentially revolutionize how organoids—3D cellular structures that emulate specific human tissue functions—are created and studied, offering new avenues for disease research and therapeutic interventions.
Advancements in Hydrogel Bio-Inks
Cai and Zhu’s original work demonstrated DASP’s potential back in 2021. However, the recent enhancements showcased in the latest publication have evolved the method into what they refer to as DASP 2.0. Utilizing “click chemistry,” which allows for rapid bonding of molecular structures, the new bio-inks introduced present a significant upgrade over prior technologies. This advancement hinges on a sophisticated multichannel nozzle, designed to mix hydrogel components just before deposition—a crucial move since the cross-linking process unfolds swiftly, transforming liquids into gels in under a minute.
A standout aspect of their development is the meticulous attention to the mechanical properties of their structures. The researchers understood that the rapid detachment of droplets from the nozzle plays a critical role in mimicking the elasticity and stiffness of human tissues. By depositing larger droplets rapidly into the matrix, they have crafted a method that promises precise control over the properties of the printed material.
Broader Implications for Medicine and Biomedical Research
The implications of Cai and Zhu’s work extend far beyond the confines of a laboratory; they herald a future where organ transplants might involve bioengineered alternatives instead of relying solely on donor organs. With DASP technology, it’s possible to envision a world where artificial tissues can be customized to fit individual patient needs, significantly reducing transplant rejection rates and enhancing recovery outcomes.
Moreover, the future applications of this methodology span across disease modeling and drug screening, offering invaluable platforms for understanding pathological processes and assessing therapeutic efficacy. By creating realistic human tissue structures, researchers can simulate disease environments more accurately than ever before, providing deeper insights into progression and treatment options.
This innovative research undoubtedly sets a new standard in the realm of bioprinting and tissue engineering. As the team continues to refine their technology, humanity stands on the precipice of transforming how we approach medicine—moving closer to a reality where organ shortages and complex diseases may become manageable, if not solvable, challenges of the future.

Leave a Reply