The pursuit of high-performance, safe, and durable energy storage solutions has increasingly focused on solid-state batteries. Among the pivotal players in this domain are lithium and sodium metal anodes, which hold promise for enhancing the electrochemical properties of batteries. However, understanding the microstructure of these highly reactive alkali metals is crucial to unlocking their potential and addressing the challenges that come with their use. Recent groundbreaking research undertaken by the Justus Liebig University Giessen (JLU), in collaboration with researchers from the U.S. and Canada, has shed light on the microstructural characteristics of lithium and sodium deposited in battery systems, paving the way for innovative design strategies.
Historically, examining the microstructure of metals has been a well-trodden path for many materials utilized in technology, as their structural characteristics directly influence their electrochemical behavior. Nevertheless, the same clarity has not been available for alkali metals such as lithium and sodium. Their chemical reactivity presents a significant barrier—their surfaces quickly react with the surrounding environment, forming reaction layers that obscure their internal structures. Consequently, it has been a longstanding challenge to analyze their microstructures, hampering advancements in battery technology that leverages these metals effectively.
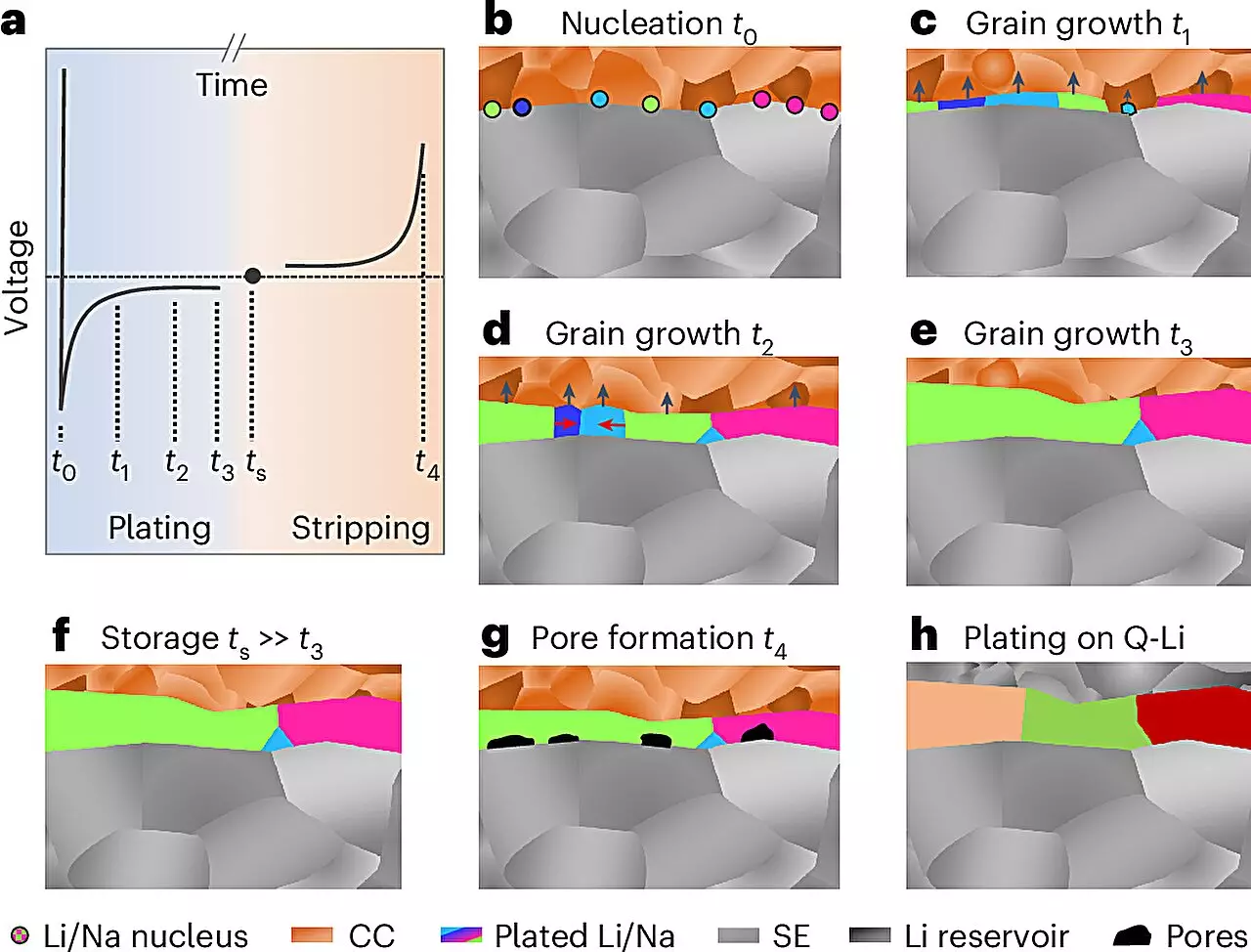
In a first-of-its-kind study, the research team from JLU, guided by the expertise of Prof. Dr. Jürgen Janek and a coalition of institutions—including the University of California, Santa Barbara, and the University of Waterloo—successfully developed a novel methodology for determining the microstructure of electrochemically deposited lithium and sodium. By employing a carefully crafted series of preparation protocols under low-temperature and inert gas conditions, the scientists utilized electron backscatter diffraction to visualize the internal structures of metal layers, a feat previously regarded as exceedingly difficult.
Their findings revealed the surprising grain size of the resultant metal layers, yielding vital insights into the foundational mechanisms underpinning their growth. According to Prof. Janek, this advancement not only enhances the understanding of lithium but also positions sodium to play a significant role in future energy storage solutions, particularly in the context of sodium battery research as part of the POLiS excellence cluster.
The implications of this research extend beyond merely mapping the microstructural landscape. Solid-state batteries, with their integration of ceramic solid electrolytes, could facilitate the effective application of lithium and sodium metal anodes in high-performance energy systems. However, several engineering hurdles remain, particularly regarding the physical stability of these metals during the charge-discharge cycle. The propensity for these metals to undergo deformation can lead to formation of pores and dendrites—structures that not only reduce battery efficiency but can also result in dangerous short circuits.
Understanding and manipulating the microstructural characteristics has potential ramifications for mitigating these issues. If lithium or sodium metal can be conditioned to prevent unwanted structural changes during electrochemical processes, one could significantly improve battery performance and safety. Researchers envision a future where these metals are preferentially formed only during the initial charging phase, alleviating concerns linked to handling and the volatility of reactive alkali metal foils.
The ongoing commitment to developing solid-state batteries has garnered extensive global research efforts over the past decade. Central to these advancements is the collaborative work being carried out by the team from JLU, which stands at the forefront of this field. Prof. Janek’s long-standing partnerships with research groups in Santa Barbara and Waterloo have yielded significant contributions to the burgeoning understanding of lithium and sodium as anodes.
The recent success of imaging the microstructure of these metals—once deemed a remarkably challenging endeavor—marks a pivotal moment in materials science. As the research team fine-tuned their approach, they crafted a pathway for future inquiries into the structural behaviors of lithium and sodium electrodes, reinforcing the importance of cross-disciplinary collaboration in achieving scientific breakthroughs.
The revelations stemming from this research not only herald a new era for lithium and sodium in the solid-state battery domain but also underscore the significance of meticulous scientific inquiry. By dissecting and understanding the microstructure of these metals, researchers are equipping themselves to enhance energy storage technologies fundamentally. As battery demands continue to rise globally, the ability to innovate and optimize will be paramount, and studies like these serve as a foundation for future advancements in energy storage design. As scientists forge ahead, the goals are clear: to create solutions that are not only efficient but also safe and sustainable, charting an exciting course for the future of energy storage.

Leave a Reply