In our daily lives, the prevalence of polypropylene (PP) is undeniable. From the containers that hold our food to the medical devices that save lives, this versatile plastic has become a cornerstone of modern manufacturing and consumer products. The essential building block for production, propylene, is derived from propane—a natural gas that many associate solely with BBQ grills. However, as the demand for polypropylene grows, so does the necessity for efficient production methods of propylene. Recent groundbreaking research from esteemed laboratories under the U.S. Department of Energy often stirs discussions about the sustainability and efficiency of petrochemical processes.
Breakthrough Research at Argonne and Ames Laboratories
Researchers at the Argonne National Laboratory and Ames National Laboratory have issued a clarion call for change by unveiling a more rapid and energy-efficient method for converting propane into propylene. This innovation could fundamentally shift the paradigm of how we approach propylene production. Their findings, soon to be published in the highly regarded *Journal of the American Chemical Society*, highlight the critical need to refine existing manufacturing processes to mitigate both economic and environmental impacts.
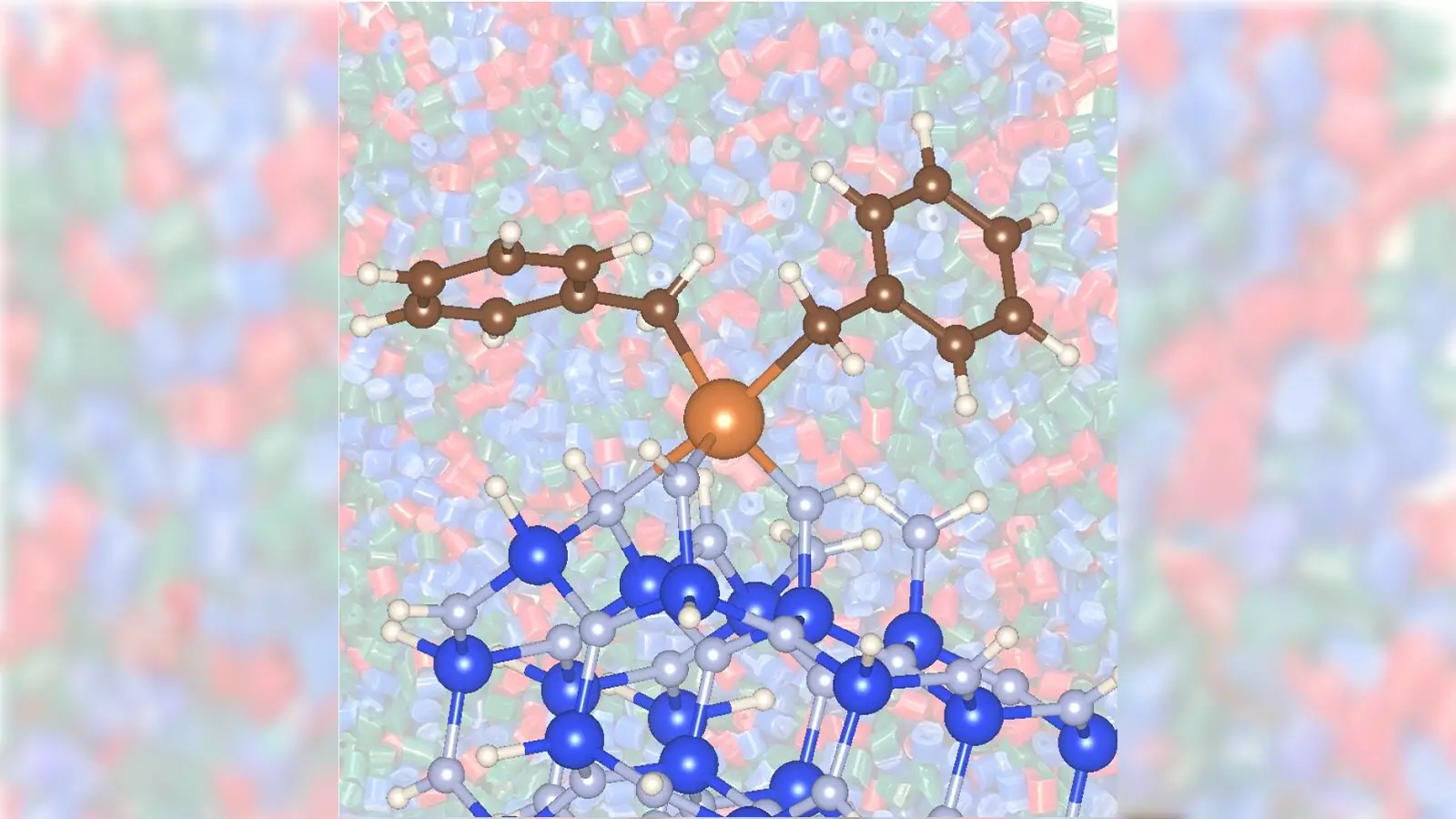
Traditionally, the conversion of propane into propylene relies heavily on metallic catalysts—often precious metals like platinum or toxic options such as chromium—utilized in high-temperature environments. These conventional methods not only demand substantial energy but often carry a greater ecological footprint due to high greenhouse gas emissions. The recent advancement employs a less toxic and cheaper variant: zirconium in conjunction with silicon nitride, facilitating a less energy-intensive reaction.
The Innovative Catalyst: Zirconium and Silicon Nitride
This novel coupling of materials represents a watershed moment in catalytic science. By utilizing zirconium on a silicon nitride support, the researchers were able to decrease the operational temperature from 1,022 degrees Fahrenheit—a standard requirement for traditional catalysts—to a more manageable 842 degrees Fahrenheit. Practically speaking, this reduction translates to significant energy savings and, crucially, a lower carbon dioxide output, which is a major contributor to global warming.
What truly stands out in this research is the demonstration that silicon nitride, as a supporting material, not only enhances the catalytic activity but does so in a manner that is both quicker and more efficient than traditional alternatives. This discovery emboldens scientists to explore the landscape of low-cost metal catalysts, expanding the toolkit available for future chemical reactions.
An Interdisciplinary Approach to Catalysis
This study is a testament to the power of interdisciplinary collaboration. Lead researchers David Kaphan and Max Delferro, along with their dedicated team, meticulously investigated how nontraditional surfaces can facilitate catalysis. Their work embodies a fundamental shift in thinking, investigating less familiar materials that could yield extraordinary results. Translation of theoretical concepts into practical applications requires a fertile ground of diverse expertise, something this research team exemplifies.
The utilization of advanced imaging techniques, such as X-ray absorption spectroscopy at Argonne’s Advanced Photon Source, allowed for a deeper understanding of the interactions between zirconium and silicon nitride. By emphasizing material characterization, the researchers shed light on previously unexplained facets of catalytic behavior, setting a new benchmark for future studies in chemical catalysis.
Implications for the Future of Green Chemistry
The significance of this research transcends mere academic interest; it points to a future rooted in sustainability and efficiency. As industries increasingly feel the pressure to reduce their carbon footprints, the evolution of chemical processes that rely on less toxic and more cost-effective materials becomes imperative. The promise of this zirconium/silicon nitride catalytic system offers multiple avenues for further investigations, not just within propane conversion but for many other critical chemical transformations.
This pioneering work also opens the door for advancements in reactivity with other transition metals. Proponents of green chemistry will find much to celebrate in this study, as it not only contributes directly to our understanding of catalysis but also offers a viable roadmap for developing environmentally responsible processes.
Acknowledgements and Collaborative Efforts
Success in this endeavor speaks volumes about the collaborative nature of contemporary science. Each researcher involved brought unique skills and insights that contributed to the overall objective. From advanced techniques in nuclear magnetic resonance to high-resolution imaging, the melting pot of knowledge proved essential.
In reflecting on the journey, the research team underscores the belief in collective effort—indicative that when experts unite, transformative results can follow. The merging of different scientific backgrounds and approaches not only enriched this study but also fortifies the field of catalytic science for future exploration and applications.
As propylene stands at the nexus of popular consumerism and increasing environmental concerns, the findings from Argonne and Ames Laboratories are a beacon of hope for a more sustainable approach to material science. This pivotal research not only challenges existing paradigms but also paints an optimistic picture for the evolving field of green chemistry.

Leave a Reply