Recent advancements in the field of catalysis have highlighted the potential of chromium-doped transition metal hydroxides in enhancing the efficiency of the oxygen evolution reaction (OER). This breakthrough is significant for several emerging technologies, particularly in the realms of water splitting and metal-air batteries, which are vital for renewable energy storage solutions. A research team, led by scientists from Tohoku University, has made strides in developing an innovative and cost-effective catalyst that could reshape the landscape for hydrogen production through water electrolysis.
The Challenge of Oxygen Evolution Reaction
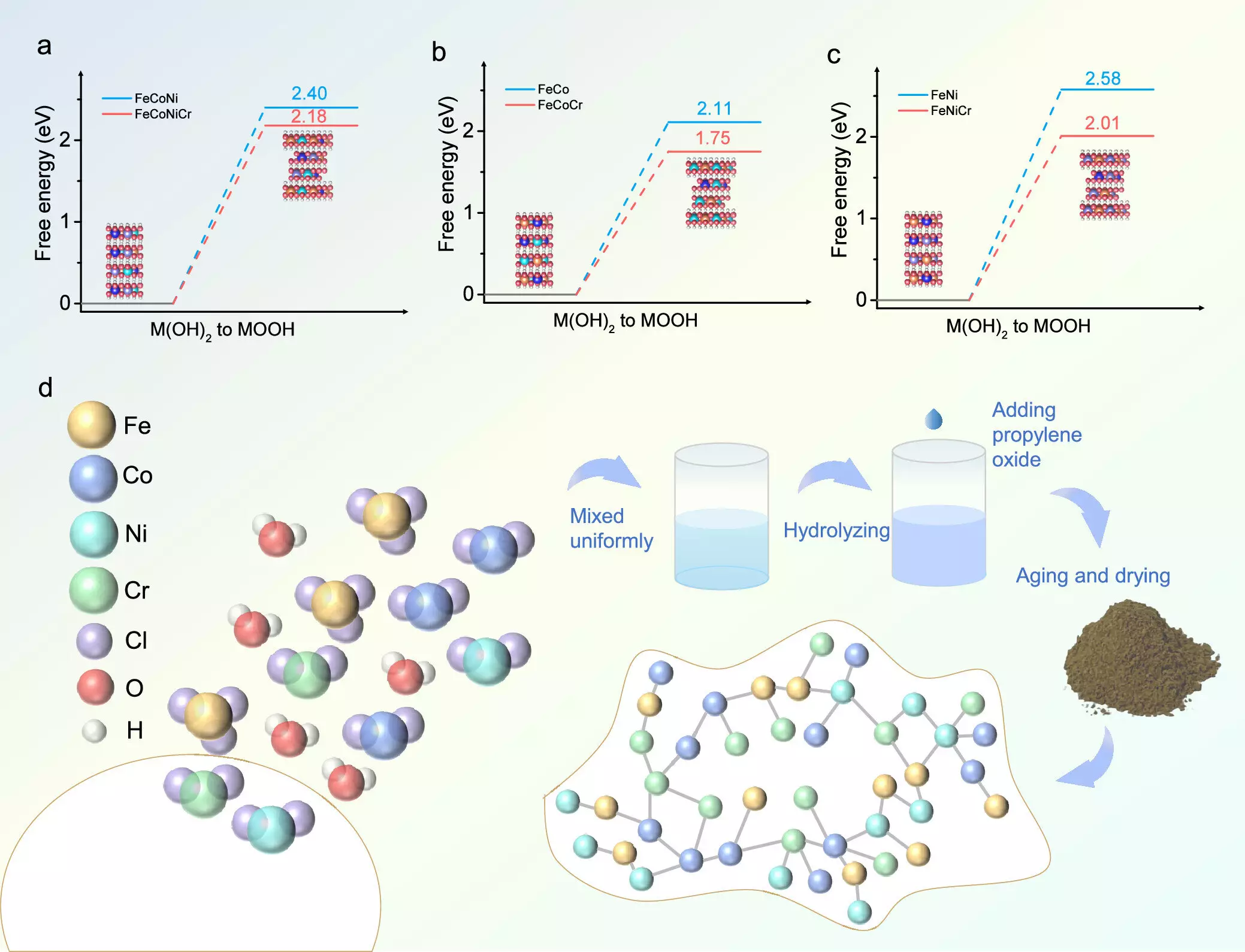
Hydrogen production via water electrolysis is a promising mechanism for storing energy derived from intermittent renewable sources such as wind and solar. However, the challenge lies in the OER, which suffers from sluggish kinetics that hamper overall efficiency. The process demands highly active and stable catalysts to facilitate faster reaction rates. Addressing this challenge, the recent study demonstrated that incorporating chromium into transition metal hydroxides could remarkably enhance catalytic activity. Researchers utilized density functional theory (DFT) alongside experimental synthesis to validate their hypotheses, marking a pivotal moment in the search for viable catalysts.
Innovation Through Synthesis
The catalyst synthesized by the team, composed of iron (Fe), cobalt (Co), nickel (Ni), and chromium (Cr), was created using an aqueous sol-gel method. This approach enabled an even distribution of the four components, resulting in a catalyst that showcased an impressive low overpotential of 224 mV in alkaline conditions. This value not only signifies a better performance compared to existing catalysts—improved by 52 mV—but also reflects sustained stability over extended use, showcasing the resilience of this new material.
In practice, this catalyst displayed remarkable performance in a zinc-air battery setup, achieving 160 hours of stable operation with minimal voltage differences during discharge and charge cycles. These results underline the potential for real-world applications of this catalyst in energy storage systems.
A deeper analysis of the catalysts revealed that chromium doping significantly optimizes the adsorption energies of intermediates involved in the OER, enhancing the overall reaction efficiency. The research team employed Bader charge analysis, which indicated a stable oxidation state for nickel and cobalt, further contributing to the sustained catalytic activity necessary for efficient hydrogen production.
Looking to the future, the researchers express ambition to explore additional elements that could further improve the catalyst’s performance metrics. The insights gained from this study offer a framework for rapidly screening prospective materials for catalyst development, representing a significant leap towards optimizing clean energy technologies.
As the world grapples with the pressing need for sustainable energy solutions, novel approaches to catalyst design will play an essential role in advancing renewable energy systems. The innovative methodologies emerging from this research not only pave the way for more efficient catalysts but also invite further exploration into the integration of corrosion-resistant materials and new compositions. The journey to achieving efficient, durable catalysts could ultimately accelerate the transition to clean energy, marking an essential step towards a greener future. Researchers remain committed to unlocking the full potential of chromium-doped catalysts, ensuring that they contribute effectively to the global quest for sustainable energy solutions.

Leave a Reply