Samarium (Sm), a rare earth element, has emerged as a pivotal player in the realm of organic chemistry, primarily due to its ability to undergo single-electron transfer reductions through its divalent compounds. Among its various forms, samarium iodide (SmI2) stands out for its moderate stability and effectiveness under relatively mild conditions, notably at room temperature. This quality renders it particularly advantageous for the synthesis of pharmaceuticals and bioactive molecules. Despite its utility, the reliance on stoichiometric amounts of SmI2 poses significant challenges, as these reactions typically necessitate substantial quantities. Coupled with the hazardous nature of some of the chemicals involved in these reactions, the process tends to be both resource-intensive and economically burdensome, thereby calling for innovative solutions that minimize the use of this precious metal.
Current strategies to mitigate the excess usage of samarium involve reducing its required quantities to catalytic levels. However, many established methods are hampered by their reliance on harsh reaction conditions and highly reactive reducing agents, still demanding 10–20% of the raw material costs in Sm reagents. Given the escalating prices of rare earth metals, the chemistry community is increasingly recognizing the urgency for a more efficient catalytic system that utilizes minimal Sm while operating under gentler conditions.
New methodologies are essential for advancing the field of organic synthesis, focusing on sustainability and cost-effectiveness. These priorities have stimulated a search for alternatives that would allow the same high-value chemical transformations with reduced environmental impacts and financial expenditure.
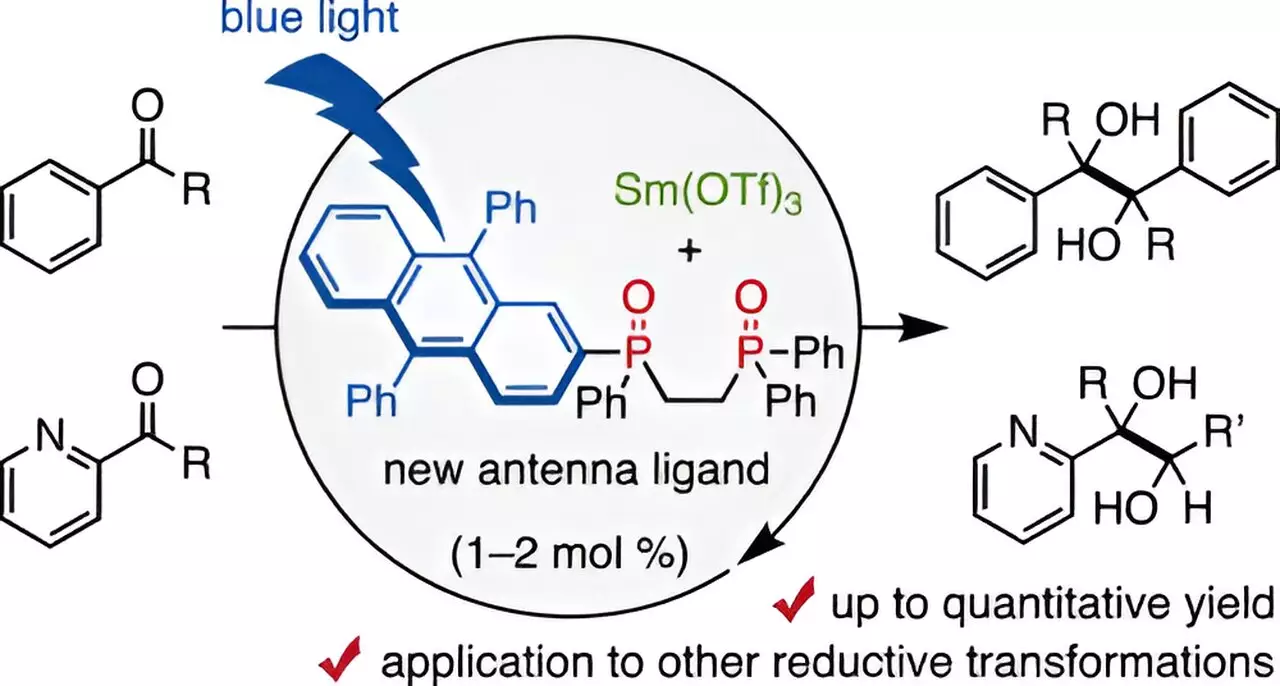
In a significant development, a team of researchers at Chiba University, including Assistant Professor Takahito Kuribara, has made strides in enhancing the efficiency of Sm-based catalysts. Their research, which has gained recognition in the Journal of the American Chemical Society, introduces an innovative ligand: a DPA-substituted bidentate phosphine oxide. Dubbed a visible-light antenna, this ligand coordinates effectively with trivalent samarium, harnessing the power of visible light to facilitate samarium-catalyzed reductive transformations.
Assistant Professor Kuribara remarks, “Antenna ligands are known to assist in the excitation of lanthanoid metals like Sm.” Using this understanding, the researchers devised a method that enables reductions to occur using merely 1–2 mol% of samarium, a remarkable decrease from the historically required stoichiometric amounts. This development underscores an important shift towards less resource-intensive methodologies in organic synthesis.
Through rigorous experimentation, the research team demonstrated that when DPA-1 interacts with blue light, it triggers effective pinacol coupling reactions of aldehydes and ketones—reactants pivotal in pharmaceuticals—yielding astonishing results, with reaction efficiencies reaching 98%. Notably, the reactions succeeded even when utilizing mild organic reducing agents like amines, a significant departure from the potent agents typically employed in such processes.
Interestingly, the inclusion of small amounts of water appeared to boost reaction yields, suggesting that reaction conditions could be fine-tuned for optimal performance. However, it was observed that excessive water could inhibit the reaction pathway, indicating a careful balance is necessary for achieving the best results.
An intriguing aspect stems from the comparative analysis of other ligands such as DPA-2 and DPA, which—despite structural similarities to DPA-1—yielded markedly poorer outcomes. This prompted further investigation into the electronic behavior of the Sm catalyst when paired with DPA-1, revealing that DPA-1 serves as a multifunctional ligand capable of both efficient light absorption and electron transfer.
The implications of this research extend beyond mere optimization of Sm usage. The ability to apply the Sm-DPA-1 combination to a wide array of chemical transformations, including crucial reactions for drug development, marks a significant advancement in the field. The versatility demonstrated, alongside the simultaneous achievement of both Sm-based reduction and photo-oxidation, showcases a promising avenue for future research in organic synthesis.
By utilizing visible light as a benign energy source, the research team has set the stage for the design of next-generation samarium-based catalysts that minimize environmental impacts while maintaining high efficiency. Assistant Professor Kuribara encapsulates the essence of this groundbreaking work: “Our new visible-light antenna ligand ensures that we can perform reductions more efficiently with minimal amounts of Sm, paving the way for sustainable practices in organic chemistry.”
As the chemistry community progresses towards a more sustainable future, this breakthrough illustrates the potential of innovative solutions in reducing dependencies on scarce materials while enhancing reaction efficiencies—truly a landmark achievement in the ongoing pursuit of greener methodologies.

Leave a Reply