Mechanochemistry—the domain where mechanical force induces chemical change—has emerged as a transformative avenue for advancing material sciences, organic synthesis, and biomedical applications. Among mechanochemical systems, mechanophores play a pivotal role: these molecules respond to physical stress by undergoing specific chemical transformations. A particularly striking example is the recently developed NEO mechanophore, designed by chemists including Jeffrey Moore and Yunyan Sun from the University of Illinois Urbana-Champaign. Unlike typical molecules, NEO can mechanically release controlled amounts of carbon monoxide, a gas with therapeutic potential. This innovative approach promises to create on-demand drug delivery systems triggered by mechanical stimuli, opening exciting avenues in medicine.
However, a fundamental challenge has long restrained progress in mechanophore design: accurately predicting when and how the carbon-carbon (C–C) bonds will break under mechanical force. These bonds, often viewed as resilient, undergo complex transformations influenced by the direction and magnitude of applied forces—rendering experimental and computational predictions time-consuming and uncertain. This unpredictability hampers efficient mechanophore design and limits the discovery of new mechanically responsive materials.
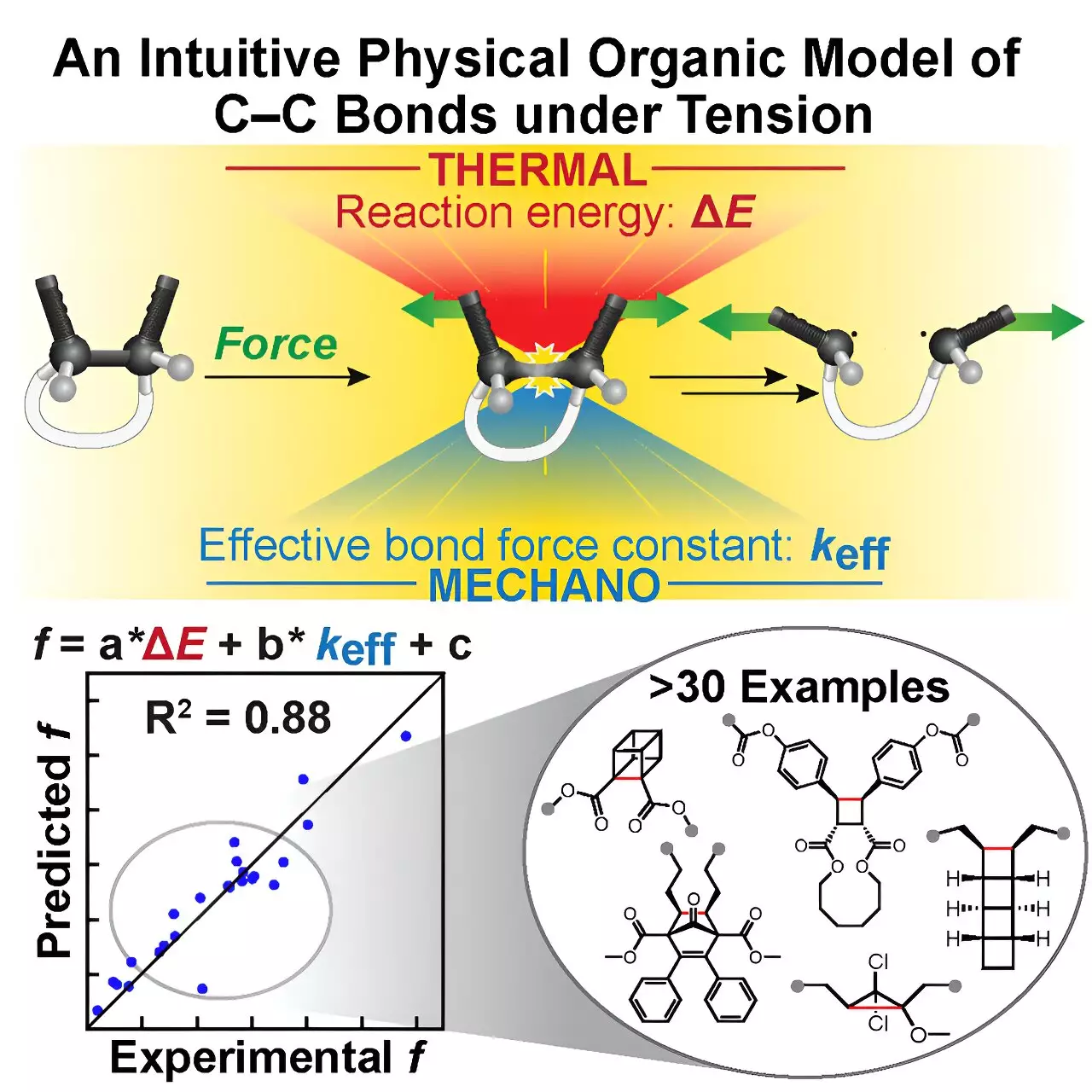
Introducing the Tension Model of Bond Activation (TMBA): Simplicity Meets Precision
Addressing this bottleneck, a collaborative effort among researchers from Urbana-Champaign, MIT, and Duke University has yielded a groundbreaking solution—the tension model of bond activation (TMBA). This novel computational framework demystifies the complicated mechanics underlying C–C bond cleavage by reducing the problem to two intuitive parameters: the effective force constant and the reaction energy. This simplicity is achieved by drawing from the Morse Potential, a classical and accessible model describing atomic interactions familiar to early chemistry students.
By representing the interplay of forces through a geometric construct—coined as the “restoring force triangle”—TMBA translates complex quantum chemical calculations into a visually and conceptually digestible form. This approach not only streamlines the prediction of when a C–C bond will rupture under tension but also provides actionable insights into designing mechanophores with desired activation profiles. The elegance of the model lies in making the chemistry accessible to a broad audience, bridging the gap between elaborate computational data and practical chemical intuition.
Why TMBA Transforms Mechanochemical Research
Traditional approaches to mechanophore design rely heavily on exhaustive experimentation or intricate computations that, while accurate, provide results that are often opaque to newcomers and even specialists. TMBA flips this paradigm by offering a mnemonic tool that one can apply rapidly to forecast molecular behavior without needing supercomputers or months of lab work. This democratization of mechanochemical analysis is a vital leap, enabling chemists to iterate mechanophore designs faster and more efficiently.
Moreover, TMBA is not just a heuristic; it has been rigorously validated against experimentally determined force thresholds and applied successfully to over thirty existing mechanophores. This robust validation underscores its predictive power and broad applicability—not limited to NEO but extendable across diverse molecular systems involving C–C bond activation.
Building Bridges Through Cross-Disciplinary Collaboration
The innovation behind TMBA epitomizes the strength of interdisciplinary collaboration. Integrating expertise from chemistry, chemical engineering, and materials science, the involved teams harnessed diverse experimental techniques and computational methods. The MONET Center for the Chemistry of Molecularly Optimized Networks facilitated this collaboration, blending academic insights with technical proficiency from multiple institutions.
Co-author Ilia Kevlishvili, a postdoctoral researcher at MIT, highlights how this synergy was essential to piece together disparate data into a unified conceptual framework. Complementing this, the Duke team, led by Professor Stephen Craig, contributed single-molecule mechanical analyses, experimentally grounding the model’s theoretical underpinnings.
The resulting framework not only accelerates current research but also serves as a foundation for future mechanochemical discoveries—by offering a common language and method for understanding and manipulating bond reactivity under mechanical stress.
Changing the Textbook Narrative: The C–C Bond Is Not Invincible
Beyond practical utility, TMBA challenges a deeply ingrained dogma in chemistry: the notion that the C–C bond is fundamentally indestructible under mechanical action. By showing predictable, controllable bond breaking events, this research invites us to rethink fundamental bond stability concepts at the molecular scale.
Jeffrey Moore envisions TMBA becoming an educational staple, a textbook concept introduced early in chemical curricula. This would revolutionize how emerging chemists conceptualize molecular force interactions—not as abstract, complex phenomena, but as accessible and predictable realities. Instilling this understanding in students would accelerate innovation by cultivating intuition alongside computational literacy.
Personal Perspective: Why This Advance Excites Me
Having pondered the limitations in mechanochemical research, I am genuinely enthusiastic about TMBA’s potential to transform both theory and practice. Often, computational models—while powerful—remain enigmatic to many researchers, limiting their practical value. TMBA’s simplicity, paired with demonstrated accuracy, hits the sweet spot of usability and rigor that the field desperately needs.
What excites me even more is the prospect of TMBA acting as a catalyst for discovering unimagined mechanophores, heralding a new era of functional materials and therapies triggered by mechanical inputs. The model distills complexity without losing nuance, offering a toolset that empowers researchers rather than overwhelms them.
TMBA is more than a model; it is an invitation to re-envision the chemistry of force. It encapsulates the spirit of scientific progress—where deep understanding paves the way for practical breakthroughs, and where interdisciplinary collaboration breathes life into innovative ideas. Mechanochemistry, with tools like TMBA in hand, is poised to unleash a wave of discovery that extends far beyond what we once thought possible.

Leave a Reply