Ethers, a class of compounds that play crucial roles in pharmaceuticals, food production, and personal care products, have long been important in the field of organic chemistry. Their versatility stems from their functional properties, making them integral in formulations ranging from medicinal drugs to consumer goods. Despite their prevalence, the conventional methods for synthesizing ethers have often been complex and inefficient, leading chemists to search for more streamlined processes. A team of researchers at the University of Illinois Urbana-Champaign has made significant strides in enhancing ether synthesis by modeling their approach after biological enzymes, heralding a new era in chemical synthesis.
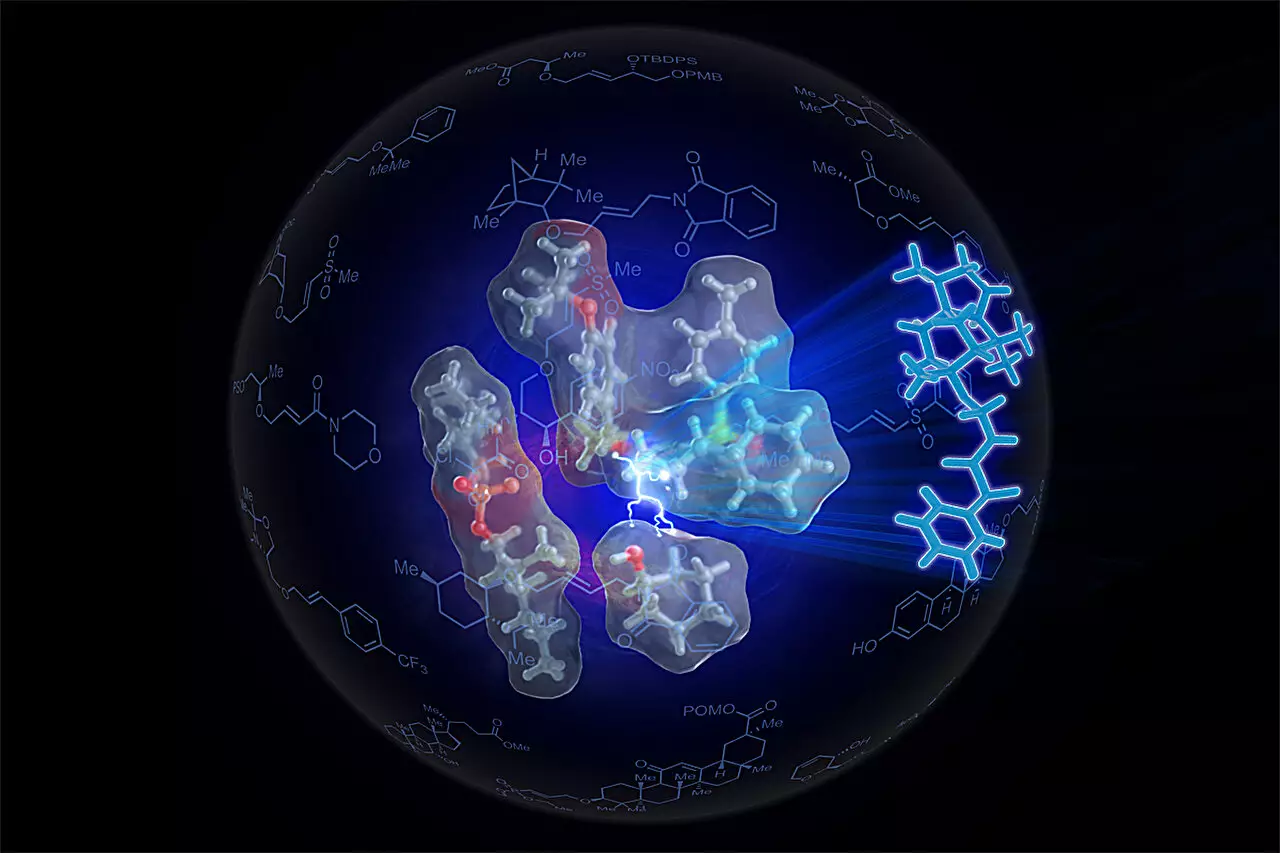
Led by Professor M. Christina White, the research group sought to harness the efficiency of nature’s own catalysts—enzymes. Enzymes facilitate chemical reactions with remarkable specificity and efficiency, often in conditions unsuitable for traditional chemical reactions. By examining how enzymes position reactants for optimal interaction, White and her team designed a new synthetic catalyst. This self-assembling small-molecule catalyst, named Sven-SOX, demonstrated the ability to replace traditional methods with a simpler and more effective protocol for producing ethers.
The challenge in ether synthesis typically lies in the requirement of particular conditions to activate the reactants for effective coupling. Standard methodologies necessitate the generation of reactive intermediates through the deprotonation of alcohols, which often leads to a multitude of by-products and the necessity of extensive purification processes. This not only wastes materials but also complicates the production of complex molecules. Sven Kaster, a graduate student and the lead author of the study, articulated the need for an innovative solution that could circumvent these hurdles.
Sven-SOX embodies a significant advancement in ether synthesis due to its unique construction. Incorporating palladium, a metal known for its reactivity, this catalyst effectively cleaves carbon-hydrogen bonds in alkenes to trigger a reaction with alcohols, forming the desired ether product without the need for aggressive activation methods. The breakthrough involves a synergy of spatial and electronic attributes, bringing reactants into close proximity and ensuring they are facing the correct orientation—a requirement analogously described as two people needing to be both close and facing each other to hold hands comfortably.
This design philosophy not only enhances the yield of desired products but also broadens the range of ethers that can be synthesized. The research team successfully produced over 130 different ethers, including intricate structures that had eluded traditional synthetic routes. The results signify that the Sven-SOX catalyst can handle complex substrates that are typically challenging to synthesize, thus expanding the toolkit available for chemical manufacturing.
What sets this new method apart is its efficiency and simplicity. It encompasses fewer steps than traditional protocols and utilizes smaller quantities of starting materials to achieve significant yields. Kaster remarked on the practicality of the procedure, suggesting that even a middle school student could comprehend and execute the synthesis. This accessibility could democratize ether production, making it feasible for a broader array of laboratories, including those with limited resources.
Furthermore, the mild reaction conditions characteristic of the Sven-SOX catalysis mean that sensitive functional groups often present in complex organic molecules can be preserved, thus enhancing the versatility of the method. Traditional methods often involve harsh conditions that can degrade these sensitive components, making this new approach a preferred option for chemists working on intricate molecular architectures.
The implications of this research extend beyond ether synthesis. The successful application of small-molecule catalysts resembling enzymatic function highlights a promising direction for future research. The team intends to explore the potential of designing additional catalysts that emulate other enzymatic processes, which could lead to significant advancements in the synthesis of various classes of chemicals.
White concluded that this breakthrough emphasizes the vital role of fundamental scientific research and invites further innovation in the development of small-molecule catalysts. The learnings from this research point toward new methodologies that could profoundly affect both academic research and industrial applications.
The marriage of chemistry and biology represented by this research paves the way for a new paradigm in organic synthesis, underlining the value of nature as a guide for scientific progress. This work not only improves existing methodologies but also inspires a fresh outlook on future chemical synthesis challenges.

Leave a Reply