In a groundbreaking development, researchers from the University of Warwick and the University of Manchester have engineered an advanced computational framework designed to refine the freezing processes crucial for medicine and vaccine preservation. The efficacy of several medical treatments—including vaccines, fertility agents, blood donations, and cancer therapies—depends heavily on effective cryopreservation, which involves rapid freezing. This necessity arises from the fact that certain treatments, if not preserved immediately, must be used right away, reducing their availability. The research team’s findings, recently published in the prestigious journal *Nature Communications*, promise a paradigm shift in how cryoprotectants are identified and utilized within the medical field.
Cryoprotectants are vital substances employed to safeguard biological materials during the freezing process. Their role is to inhibit ice crystal formation, a challenge that significantly compromises the integrity and availability of medicines once thawed. While existing cryoprotectants serve to protect cells, many fail to effectively prevent ice crystal growth, which is a critical factor during both freezing and thawing. In recognizing this limitation, the research team embarked on an innovative journey to discover new cryoprotectants that could address this gap.
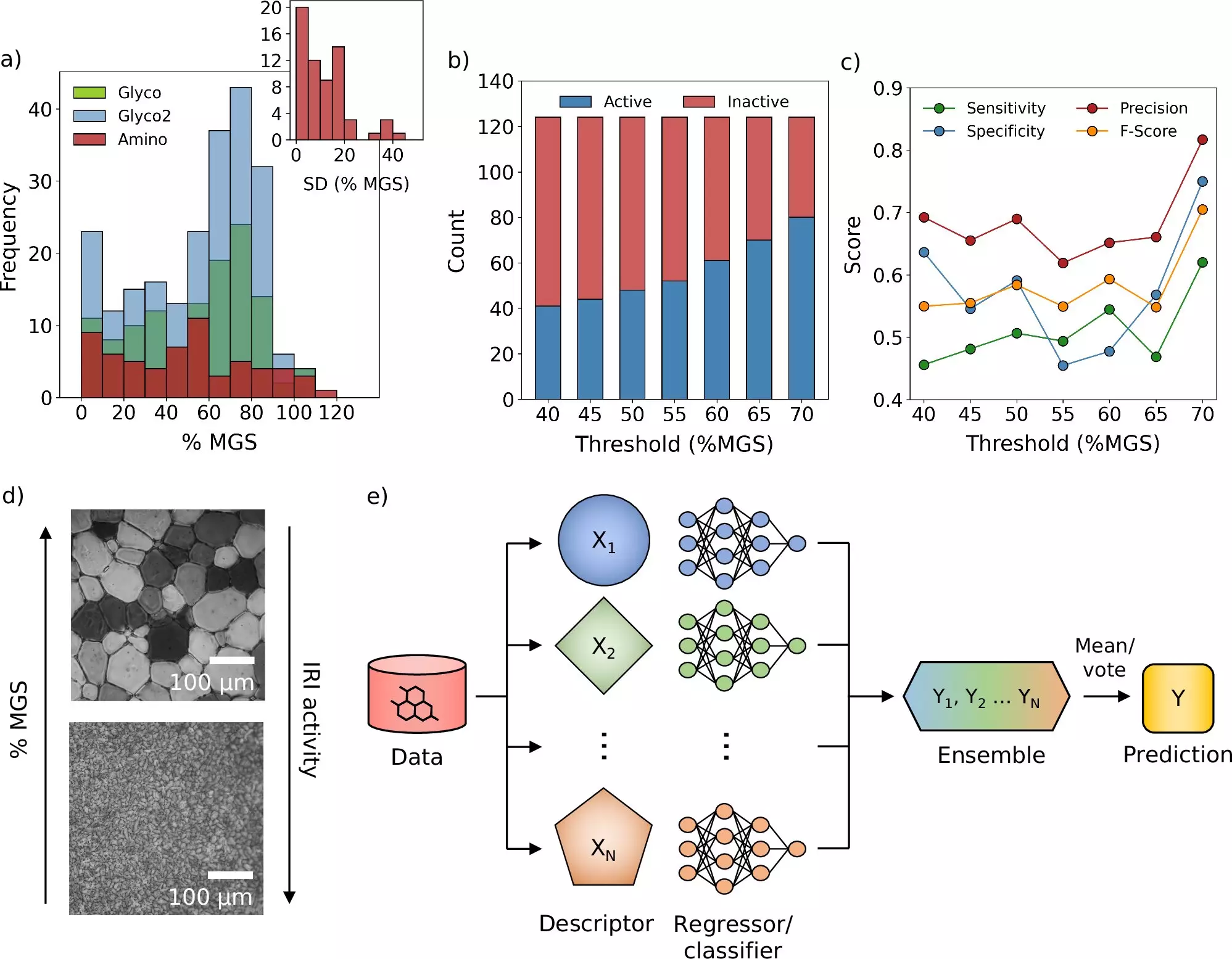
Professor Gabriele Sosso, who spearheaded the study at Warwick, highlighted the role of machine learning in this endeavor. He emphasized that while machine learning is not a panacea for every scientific hurdle, employing it synergistically with traditional molecular simulations and laboratory experiments was key to their success. This multifaceted approach allowed the team to harness the capabilities of machine learning to streamline their research processes, significantly refining the method of cryoprotectant discovery.
The researchers developed a sophisticated computer model that achieved the unprecedented task of sifting through vast chemical libraries, identifying candidate molecules that could serve as potential cryoprotectants. This marks a critical departure from the conventional trial-and-error methodology, which has long hampered research progress, delaying timely medical advancements. The model is not just a theoretical innovation; it facilitated the identification of a novel molecule that effectively curbs ice crystal growth—a paramount challenge in cryopreservation.
Dr. Matt Warren, a Ph.D. student who played a significant role in the project, expressed his enthusiasm with the transition from extensive laboratory data collection to a robust machine-learning framework. He highlighted how this tool enables a predictive approach to assessing cryoprotective activity, thus accelerating scientific inquiry while freeing up valuable time for researchers to tackle intricate problems that still necessitate human creativity and expertise.
What’s particularly noteworthy about this research is its real-world application; the team successfully demonstrated the potential to reduce the quantity of traditional cryoprotectants required for blood storage by introducing their newly discovered molecules. This could lead to a swifter blood washing procedure post-freezing, expediting the transfusion process and ultimately enhancing patient care. The implications extend beyond immediate medical applications; the research team’s findings could also pave the way for discovering more efficient cryoprotectants and repurposing existing molecules.
Professor Matthew Gibson from the University of Manchester shared insights from his extensive experience studying ice-binding proteins in polar fish, indicating that their research has traditionally met with slow progress. However, the collaboration with Professor Sosso has not only revitalized but also transformed their research efforts, as the computer model yielded unexpected yet promising candidate molecules, illustrating the immense potential tied to interdisciplinary collaborations.
The intersection of machine learning and traditional scientific methods heralds a new frontier in cryobiology, with profound implications for the medical field. As this innovative technology evolves, it holds the promise of revolutionizing how we think about and implement preservation techniques. The collaborative efforts of these researchers stand as a testament to the power of blending interdisciplinary expertise to overcome longstanding challenges in science, paving the way for improved medical treatments and enhanced patient outcomes. As we look ahead, the potential for future breakthroughs in how we preserve life-saving medications and therapies shines brightly, driven by the continuous embrace of technological advancements.

Leave a Reply