In the evolving realm of chemical synthesis, one term has repeatedly emerged: click chemistry. Recognized for its robustness and efficiency, click chemistry allows for the strategic linking of molecules with remarkable speed and selectivity. This innovative approach has permeated various disciplines, from pharmaceuticals to materials science, owing to its high yields and minimal reactions’ complexity. However, traditional methods employed in synthesizing vital compounds often come with environmental hazards. A notable example is the synthesis of sulfonyl fluorides, pivotal in many applications. Recent advances propose a groundbreaking method addressing both efficiency and environmental safety.
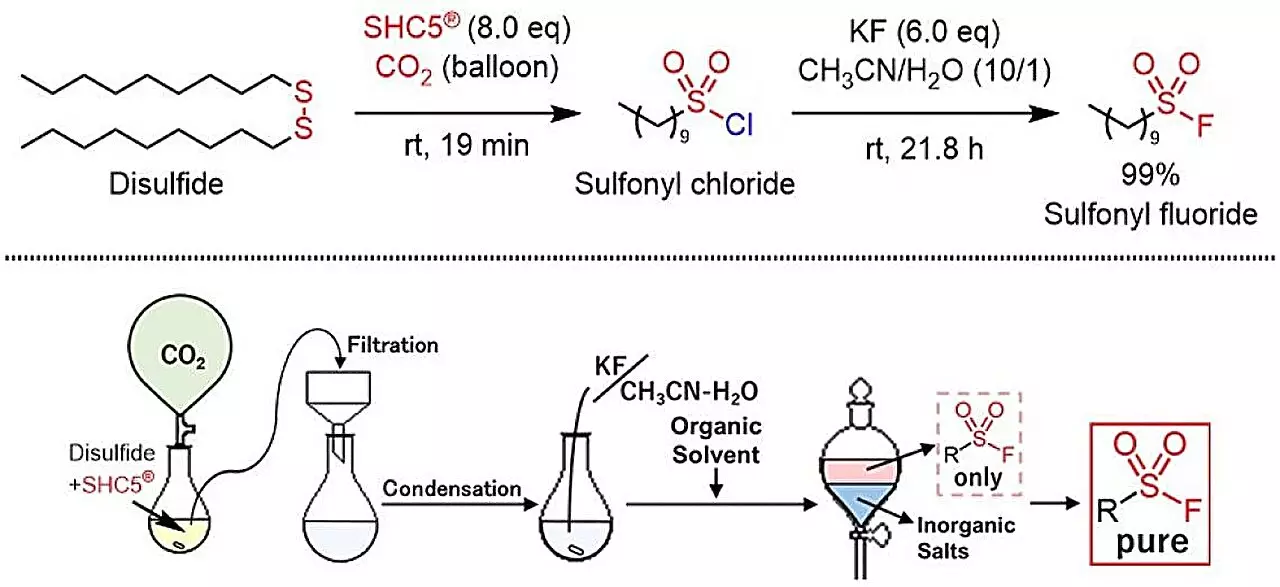
Traditionally, synthesizing sulfonyl fluorides relied on toxic reagents such as SO2F2 gas or KHF2, posing major risks in laboratory and industrial settings. A recent study published in ACS Sustainable Chemistry & Engineering introduces a transformative approach that pivots from these hazardous compounds. Researchers, led by Masayuki Kirihara, Shinobu Takizawa, and Mohamed S. H. Salem, report that by employing SHC5 and potassium fluoride (KF) in a reaction with thiols or disulfides, sulfonyl fluorides can be synthesized efficiently. This method not only showcases the potential for safer chemical processes but also emphasizes the necessity of adopting green chemistry principles.
One of the most striking features of this new chemistry protocol is the environmental consideration it entails. Unlike previous methods that released toxic gases or hazardous by-products, this innovative approach yields only sodium chloride (NaCl) and potassium chloride (KCl)—safe compounds that pose minimal ecological risk. The reaction’s design exemplifies a green synthetic process where the focus is on sustainability without compromising the yield or efficiency of the outcome. Such by-products underscore the researchers’ commitment to creating chemical methods that align with sustainable development goals, which aim to balance industrial progress with environmental stewardship.
The implications of this work extend far beyond academic interest; they pose significant potential for industrial applications. The newly developed method encourages the large-scale synthesis of sulfonyl fluorides, facilitating the production of various compounds containing aromatic, aliphatic, and heterocyclic groups. This versatility positions the method as a key player in chemical manufacturing processes. Its simplicity translates to lower costs and reduced complexity, which can significantly enhance productivity in commercial settings.
The emergence of this efficient and environmentally benign method for synthesizing sulfonyl fluorides represents a pivotal moment in the field of chemistry. As researchers continue to prioritize sustainable practices, methodologies like the one introduced in this study could pave the way for a greener future in chemical manufacturing. It embodies the principles of innovation combined with responsibility, ensuring that the progress we achieve does not come at the expense of our planet. The scientific community stands at the threshold of a new paradigm, and as this work exemplifies, the future of chemical synthesis will likely be defined by our ability to integrate sustainability into every reaction.

Leave a Reply