Carboxylic acids stand at the forefront of organic chemistry, playing a pivotal role in numerous applications, including pharmaceutical development. Compounds like aspirin and ibuprofen exemplify the necessity of this class of organic molecules in modern medicine. Their multifunctional properties make them indispensable, yet their inherent characteristics can limit their effectiveness. Thus, the quest for enhancing these compounds through innovative methods, particularly through the incorporation of fluorine atoms, emerges as a significant area of research.
The Challenge of Fluorination
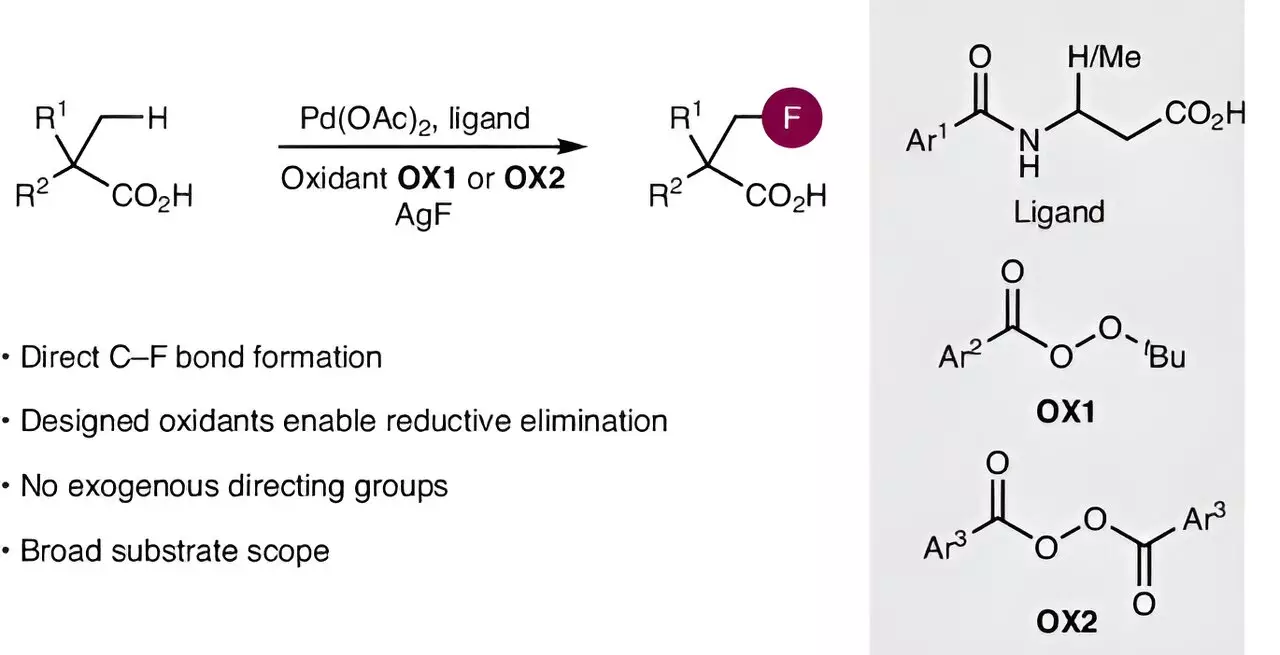
Despite fluorine’s diverse applications, integrating it into carboxylic acids has traditionally been hampered by complex synthesis pathways. These multi-step processes not only demand significant time and resources but also possess a high risk of generating by-products. The intricacies involved in activating inert carbon-hydrogen bonds for direct fluorination have long posed a major challenge. Conventional strategies often fall short, underscoring a pressing need for a simplified approach that prioritizes efficiency without sacrificing selectivity.
Innovative Strategies from Kiel University
A recent publication in *Nature Synthesis* by a collaborative team from Kiel University has unveiled a groundbreaking methodology that promises to transform how fluorine atoms can be introduced into aliphatic carboxylic acids. Under the leadership of Professor Manuel van Gemmeren, the research has materialized from a foundation built on previous studies targeting the activation of carbon-hydrogen bonds using palladium catalysts. The team’s endeavor to refine catalyst efficiency culminated in a novel catalyst design that significantly enhances the reaction’s feasibility.
Addressing Complexities with a Two-Pronged Approach
What sets this research apart is its dual-faceted approach; not only does it involve enhancing the catalyst, but it also innovatively addresses the challenge of carbon-fluorine bond formation. The researchers ingeniously developed a new oxidizing agent that facilitates this crucial reaction step. This strategy taps into an unusual reaction pathway, delivering enhanced selectivity and efficiency in the formation of carbon-fluorine bonds, which had previously remained elusive for aliphatic carboxylic acids.
Implications for Pharmaceutical Research
The implications of this novel method extend far beyond academic curiosity; they hold extraordinary promise for pharmaceutical research and development. Given the critical role that both carboxylic acids and fluorinated compounds play in drug formulation, the capability to introduce fluorine atoms directly into complex molecules without undergoing cumbersome synthesis represents a significant leap forward. The research team believes that their findings could be applied to other synthetic methodologies, setting the stage for broader impacts across various chemical applications.
A Vision for the Future
Professor van Gemmeren’s affirmation of the exceptional potential of their method resonates strongly within the scientific community. As more researchers embrace innovative approaches to chemical synthesis, the future holds a wealth of opportunities for the development of next-generation pharmaceuticals. This breakthrough could herald a new era in organic chemistry, where the integration of fluorine into compounds becomes less of a labor-intensive endeavor and more of a streamlined innovation, ensuring that drug development can keep pace with the ever-evolving challenges of modern medicine.

Leave a Reply