Recent advancements in electrochemical technology have presented opportunities to transform the landscape of chemical production, making it cleaner and significantly more energy-efficient. At the forefront of this innovation is a team from Lawrence Livermore National Laboratory (LLNL), which has pioneered a method utilizing thin film nickel anodes for chemical synthesis. This approach not only addresses the pressing issues of energy inefficiency in current chemical processes but also underscores the importance of sustainable practices in the industry.
The crux of LLNL’s research lies in the application of thin film catalysts. These nickel anodes provide a uniform surface that minimizes complications commonly associated with irregular or porous materials. As noted by postdoctoral researcher Aditya Prajapati, this consistency allows researchers to isolate and study the true catalytic properties of the material. Such clarity is crucial for enhancing our understanding of the underlying electrochemical reactions, which has direct implications for improving efficiency in chemical processes. Utilizing thin films streamlines experimentation and fosters innovation in catalyst development.
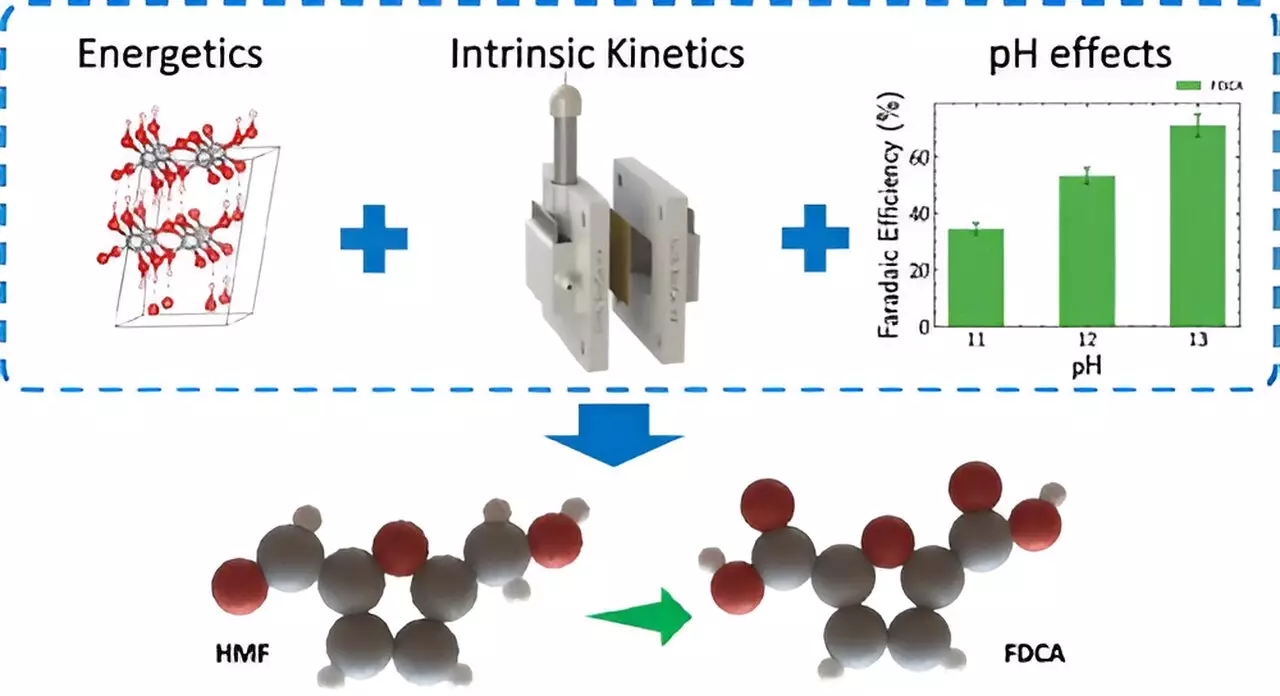
Traditional electrolyzers are known for their suboptimal energy conversion rates, particularly during the oxygen evolution process at the anode. However, the LLNL team has proposed a groundbreaking alternative: replacing the oxygen evolution reaction with biomass oxidation. This innovative shift has the potential to decrease energy consumption by over 50%, which is a remarkable feat in an age where energy efficiency is paramount. By using renewable resources such as biomass, the researchers are not only improving energy usage but are also aligning the process with broader sustainability goals.
The electrochemical method developed by LLNL facilitates the conversion of 5-Hydroxymethylfurfural (HMF), a compound harvested from biomass, into 2,5-Furandicarboxylic acid (FDCA). FDCA is increasingly recognized as a crucial building block for bio-based plastics, such as polyethylene furanoate (PEF). The implications of this transformation extend beyond mere chemical synthesis; it represents a significant step toward reducing dependence on fossil fuels and curtailing carbon dioxide emissions. This process not only aligns with the global need for sustainable alternatives but also fosters a circular economy by utilizing renewable feedstocks.
Ultimately, the electrochemical oxidation of HMF to FDCA introduces a more sustainable model of chemical production. The reduced energy demands of this method, especially when combined with renewable electricity sources, could even pave the way for achieving a zero-carbon footprint. The collaborative nature of this research, which also includes contributions from esteemed institutions like Université de Montréal and the University of Bonn, highlights the importance of generating global partnerships in addressing climate change and resource depletion challenges.
As the world seeks to balance industrial growth with ecological responsibility, advancements like those from LLNL are not just beneficial but necessary. The journey toward a cleaner, more efficient chemical production landscape has begun, and it is innovations such as these that will guide it toward a more sustainable future.

Leave a Reply