The quest for sustainable and energy-efficient practices in chemical manufacturing has become a focal point of contemporary research, particularly in regard to biomass conversion. A recent study conducted by researchers at Kyushu University has illuminated a promising path forward by showcasing the efficacy of a zeolite material known as Na-ZSM-5 in enhancing the conversion of biomass into olefins. These olefins serve as crucial precursors for an array of products, including plastics, pharmaceuticals, and food additives. By employing microwave heating, this innovative process not only challenges traditional methods but potentially mitigates the environmental impact commonly associated with chemical synthesis.
Historically, one of the primary methods for producing essential chemicals involves naphtha reforming, a process notorious for consuming vast amounts of energy and releasing significant quantities of carbon dioxide. As the chemicals produced often come from fossil fuels, this method poses a considerable challenge for sustainability. In response, researchers have turned their attention to alternative feedstocks such as cooking oil waste and microalgal oils, which can be economically viable. However, the challenge remains to convert these feedstocks efficiently.
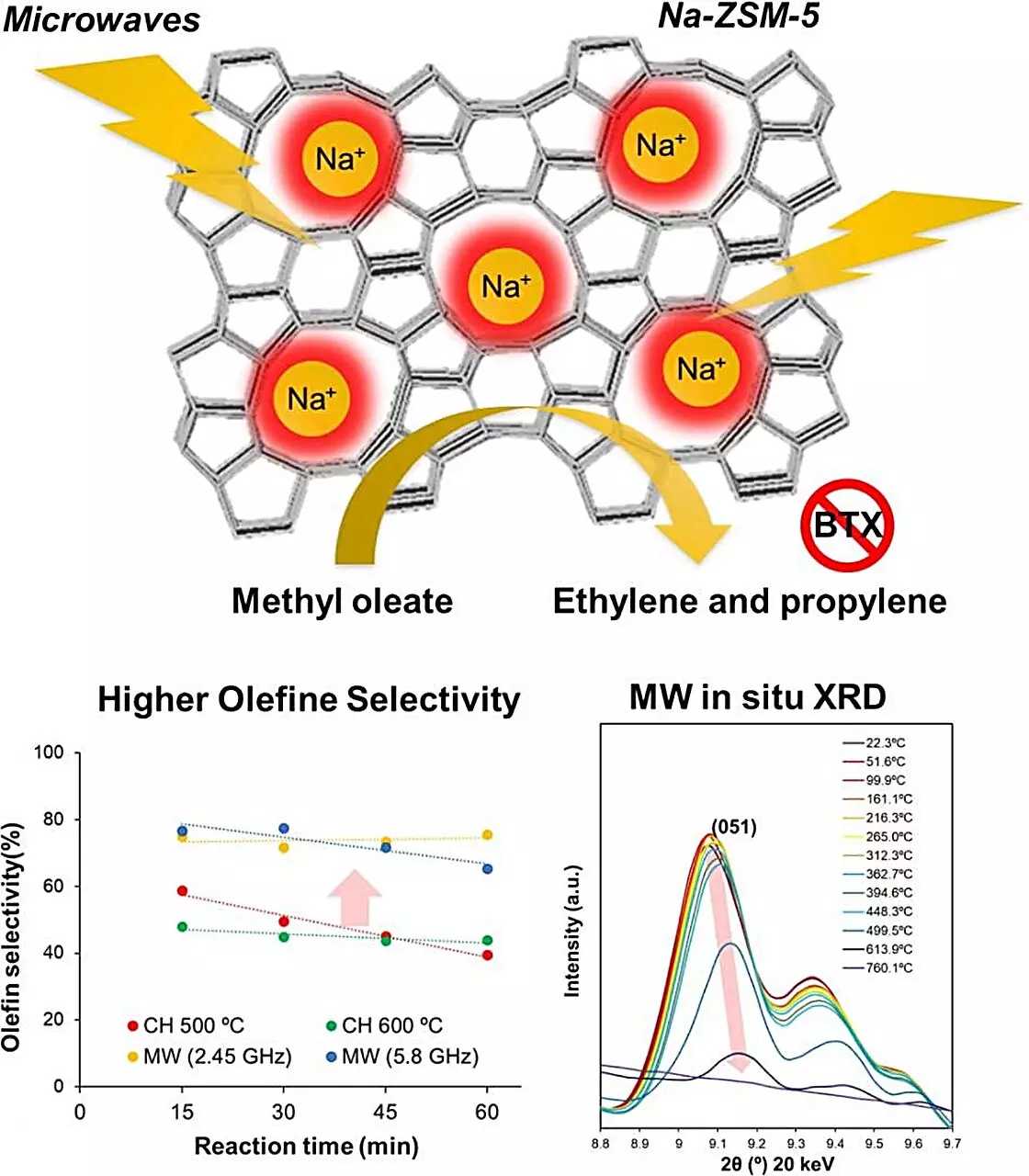
Catalytic cracking, a prevalent method in this domain, necessitates high temperatures ranging from 500 to 600 degrees Celsius, a requirement that both intensifies energy use and fosters coking—a deterioration of the catalyst caused by unwanted deposit buildup. Such limitations prompt the need for more effective and sustainable solutions.
At the heart of this groundbreaking research is the introduction of microwave heating as a pivotal enhancement to traditional catalytic processes. Associate Professor Shuntaro Tsubaki and his team explored the feasibility of utilizing microwaves to heat zeolite catalysts without incurring the adverse effects typically associated with high-temperature processes. Microwaves present a unique opportunity, as they can directly interact with materials, delivering energy selectively. This capability not only reduces energy expenditure but also minimizes the formation of coking.
Tsubaki elucidates this principle: “Microwaves interact directly with materials and can selectively deliver energy to them, enabling significant energy savings compared to conventional heat-convective processes.” This innovative approach allows for the highly precise heating of Na-ZSM-5, resulting in dramatic improvements in conversion rates.
In their rigorous experimentation, the researchers commenced by comparing multiple zeolite catalysts to determine which would be most effectively heated by microwave energy while concurrently delivering strong catalytic performance. Their analysis led to the selection of Na-ZSM-5, a sodium ion-substituted zeolite, which they then subjected to various heating methods.
Results were unequivocal: the microwave-assisted catalytic conversion of methyl oleate to olefins resulted in a conversion efficiency that eclipsed that achievable through traditional heating methods. Significantly, under microwave irradiation, CO2 emissions were suppressed to a mere 1.3% of the total output, with no carbon monoxide produced at all. Most strikingly, the olfins production with microwave heating at 500 degrees Celsius was quadrupled compared to results achieved using standard methods, showcasing Na-ZSM-5’s unparalleled selectivity.
Furthermore, the absence of coke formation at these elevated temperatures underscores the superior performance of microwave-assisted processes. Notably, the research team discovered localized temperatures within the zeolite’s crystal structure exceeding 1000 degrees Celsius, a finding that helps explain the enhanced selectivity for olefin production.
The implications of these findings are profound for the future of chemical manufacturing. By significantly improving catalytic biomass conversion, microwave heating embodies a tactical shift towards achieving sustainability in the chemical industry. The prospect of integrating microwaves generated from renewable energy sources, such as solar and wind, aligns seamlessly with the ongoing movement towards environmentally-conscious practices in the sector.
Tsubaki emphasizes the potential of their research by stating, “Our findings are expected to contribute to the further electrification of the chemical industry.” This forward-thinking approach not only heralds a new era of eco-friendly chemical synthesis but also opens avenues for additional research to scale up microwave-driven catalytic processes further.
With Kyushu University’s revolutionary discoveries at the forefront of microwave-assisted biomass conversion, the field stands on the precipice of radical change. As researchers continue to refine and enhance these methodologies, the shift towards sustainable chemical practices that minimize energy consumption and environmental impact becomes increasingly attainable. This work not only signifies a pivotal breakthrough for academic research but also paves the way toward a more sustainable future in chemical manufacturing that aligns with global ecological goals.

Leave a Reply