Ammonia (NH3) is integral to both agriculture and industrial sectors, with its production underpinning fertilizer creation and various chemical processes. The global demand for ammonia is staggering, with production volumes approximating 175 million metric tons and monetary valuations reaching around $67 billion. In an era increasingly focused on sustainability and reducing carbon footprints, the traditional methods of ammonia synthesis, particularly the Harber-Bosch process, face scrutiny due to their energy-intensive nature and substantial carbon dioxide emissions. However, recent advancements in electrochemical methods may offer a transformative pathway to producing ammonia more sustainably.
Recent scholarly contributions led by researcher Hao Li at Tohoku University’s Advanced Institute for Materials Research have illuminated an innovative route for ammonia production through the electrochemical reduction of nitrate (NO3–) instead of relying on nitrogen gas (N2) as a feedstock. The approach hinges on nitrate’s favorable properties, which include lower dissociation energy and greater solubility in water compared to molecular nitrogen. This alteration in the nitrogen source could redefine the landscape of ammonia synthesis, particularly as it potentially mitigates the environmental issues associated with nitrate leaching into water bodies.
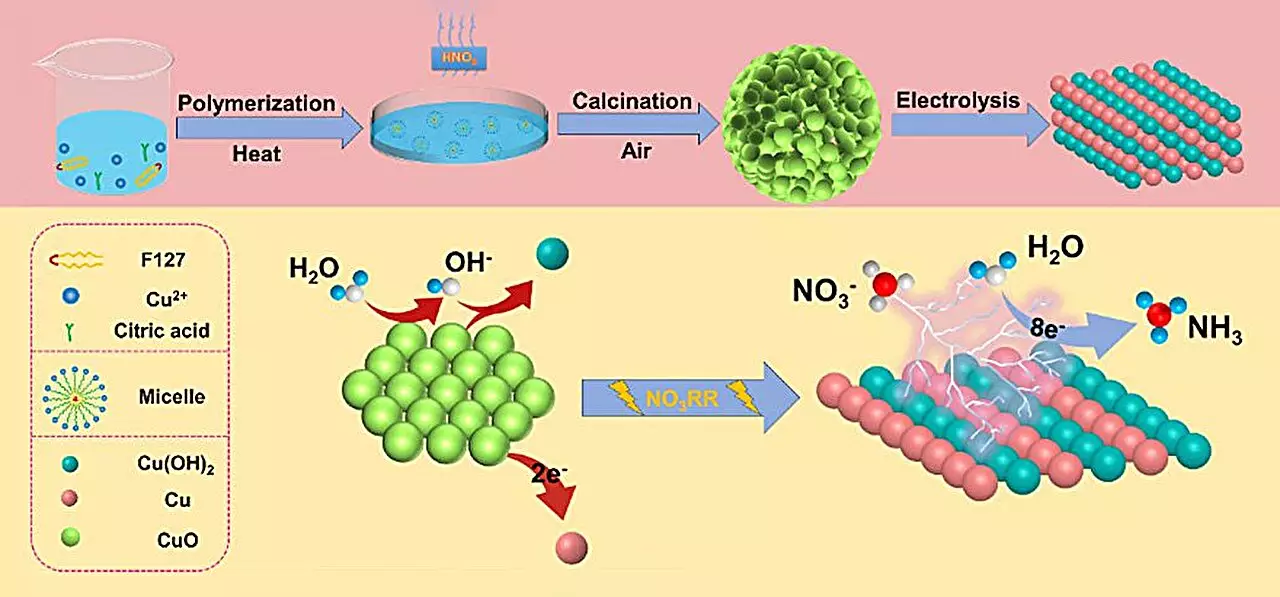
A key component of this new methodology involves the deployment of a copper (II) oxide (CuO) catalyst. The research team found that synthesizing this catalyst in a specific microstructure resulted in significantly enhanced yields of ammonia. The experiment demonstrated a remarkable output of 15.53 mg h-1 mgcat-1 and achieved a Faraday efficiency of 90.69% at a controlled voltage of -0.80 V. These findings are indicative of the catalyst’s remarkable effectiveness, which can be attributed to its unique structural properties and the transformative phase changes that occur during the reaction.
The investigation delved into the mechanics behind Catalytic Activity, emphasizing that a critical transformation from CuO to a Cu/Cu(OH)2 configuration occurs during the electrochemical reduction process. This transformation is pivotal as it not only amplifies the number of active sites available for reaction but also augments the efficiency of electron transfer at the electrode’s surface. Theoretical models utilizing density functional theory (DFT) were employed to probe deeper into these processes, revealing that the formation of the Cu(OH)2 phase effectively lowers the energy barrier for nitrate adsorption. This presents a favorable energetic profile for ammonia production while simultaneously suppressing the competing hydrogen evolution reaction.
Li and team’s findings signal a paradigm shift in the design of copper-based catalysts for ammonia synthesis via electrocatalysis. The research underscores how timely manipulation of reaction parameters and an in-depth understanding of phase-specific transformations can lead to substantial improvements in catalytic performance. The promising results not only enhance the efficiency of nitrate reduction reactions but also provide avenues for making this process scalable and efficient, potentially aligning with the wider goals of sustainable industrial practices.
As the research trajectory unfolds, there is a clear focus on refining these catalytic systems further. Understanding the variables influencing phase transitions during the reduction processes will be essential to improving the performance of these catalysts. With ongoing exploration, this innovative method could serve as a cornerstone for the sustainable production of ammonia, addressing both environmental concerns and the pressing demand for ammonia in global markets.
The expedition into the realm of electrochemical nitrate reduction heralds a promising shift in ammonia production practices. With the challenges presented by conventional methods and the intricate balance between efficiency and environmental stewardship, the avenues explored by Li and his colleagues may pave the way for a more sustainable future in ammonia synthesis. As research continues to evolve in this space, one can remain hopeful for innovations that harmonize industrial growth with environmental responsibility.

Leave a Reply