In an age where antibiotic resistance poses one of the gravest threats to global health, the emergence of innovative solutions is not just welcome—it’s essential. Recent research from the University of Illinois Chicago has unveiled a groundbreaking class of synthetic antibiotics known as macrolones, which are designed to combat bacterial infections in a remarkably ingenious manner. By targeting two distinct cellular processes, macrolones could potentially make it up to 100 million times more difficult for bacteria to evolve resistance, marking a monumental milestone in the battle against antimicrobial resistance.
Understanding the Mechanism of Action
What sets macrolones apart from traditional antibiotics is their dual-action mechanism. Unlike conventional antibiotics that operate through a single pathway, these synthetic drugs disrupt bacterial functioning in two ways: one, by inhibiting protein synthesis; and two, by altering DNA structure. This two-pronged approach does not simply complicate the bacteria’s defensive strategies—it fundamentally alters the landscape of antibiotic efficacy. The logic is both straightforward and profound: for bacteria to develop resistance, they would need to simultaneously counteract alterations to both their ribosomes (the sites of protein synthesis) and their DNA.
Alexander Mankin, a distinguished professor at UIC and a key figure in this research, underscores the significance of this dual-target strategy. “The beauty of this antibiotic is that it kills through two different targets in bacteria,” he notes, encapsulating the potential of macrolones in a single, eloquent phrase.
The Science Behind Macrolones
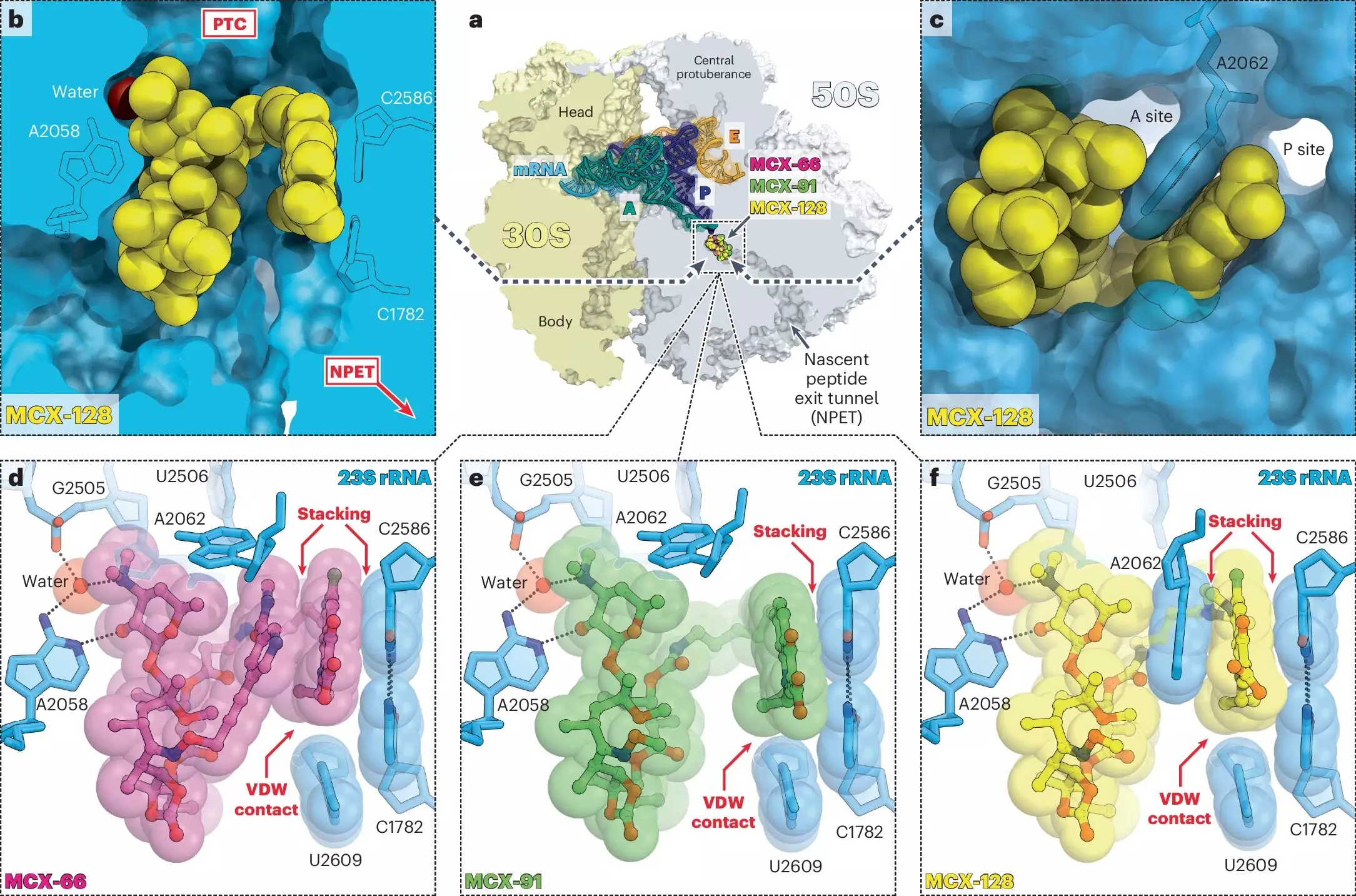
Delving deeper into the biochemical interactions, researchers from two UIC laboratories led by Mankin and Yury Polikanov have thoroughly examined how these drugs engage with bacterial systems. Macrolones ingeniously amalgamate the structural components of two well-known antibiotics: macrolides, which hinder ribosomal function, and fluoroquinolones, which target DNA gyrase enzymes crucial for DNA replication. This fusion not only enhances efficacy but also broadens the therapeutic scope against various bacterial strains.
Polikanov’s group, specializing in structural biology, has made significant strides in elucidating the binding affinities of macrolones compared to traditional macrolides. Their studies reveal a noteworthy capability: macrolones can effectively bind to and inhibit ribosomes from bacterial strains that have developed resistance to older macrolides. Crucially, these new agents do so without triggering the activation of known resistance mechanisms, providing an edge that conventional antibiotics struggle to maintain.
The Promise of Dual Targeting
Through a series of rigorous experiments, the UIC team discovered that while many macrolone designs excelled in targeting either ribosomes or DNA gyrase, one standout compound showcased the ability to inhibit both at its lowest effective dose. This discovery not only solidifies the macrolone’s potential effectiveness but also highlights an innovative strategy to circumvent bacterial adaptive mechanisms. As Mankin articulates, by addressing two targets simultaneously, the likelihood of bacteria evolving a simple genetic defense diminishes dramatically.
This dual-act nature of macrolones does not just represent a scientific achievement; it signifies a paradigm shift in antibiotic development. Researchers and chemists must prioritize the optimization of such compounds, ensuring that future antibiotics become not only effective against current strains but also remain viable options against evolving pathogens.
The Intricacies of Interdisciplinary Collaboration
The UIC study is not merely a demonstration of scientific prowess; it epitomizes the power of interdisciplinary collaboration. The Molecular Biology Research Building fosters an environment where chemists, biologists, and pharmacologists converge, promoting innovative ideas that emerge from shared expertise. This approach is critical in addressing complex issues such as antibiotic resistance, where solutions often lie at the intersection of multiple scientific domains.
As articulated by Mankin, the findings compel chemists to rethink antibiotic design with an emphasis on targeting multiple bacterial processes simultaneously. The implications of this guidance could steer future research toward other innovative compounds, reinforcing the notion that disrupting bacterial functions is key to developing effective antibiotics.
As researchers navigate the intricate landscape of antibiotic resistance, macrolones represent a beacon of hope, illustrating how scientific ingenuity and collaboration can yield the next generation of life-saving therapies.

Leave a Reply