A groundbreaking development in hydrogen production has emerged from a collaboration involving leading experts in materials science and engineering at Pohang University of Science and Technology (POSTECH) and Seoul National University. Spearheaded by Professor Jeong Woo Han and his team, including Professor Yong-Tae Kim, Dr. Sang-Mun Jung, and MSc student Yoona Kim, this research tackles a significant challenge that has the potential to enhance the efficiency of alkaline water electrolysis systems. Published recently in the journal *Advanced Functional Materials*, their findings not only shed light on overcoming the limitations of traditional catalysts but also propel the hydrogen economy forward in a sustainable manner.
The Challenge of Energy Intermittency
The transition to renewable energy sources such as wind, solar, and hydro has been lauded for its capacity to mitigate climate change. However, it presents unique challenges due to the intermittent nature of these energy supplies. Weather fluctuations can disrupt the generation of electricity, making it essential to design robust systems for energy storage and delivery. Hydrogen serves as a critical component in this energy storage puzzle; it can be produced via water electrolysis, wherein water is split to release hydrogen.
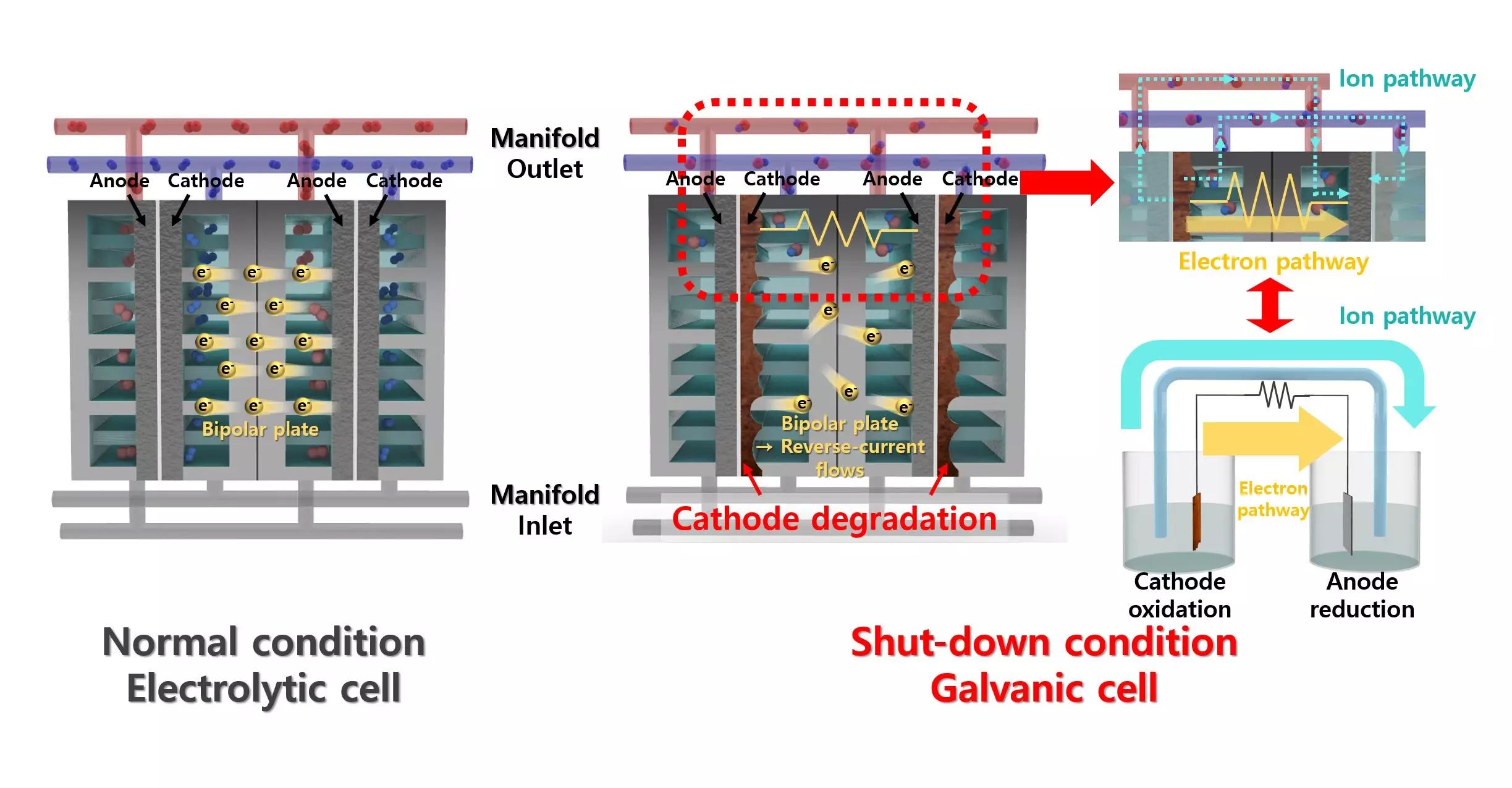
Alkaline water electrolysis (AWE) has emerged as a favorable process due to its cost-effectiveness and durability. Yet, the inherent instability of the energy supply introduces a significant problem: the degradation of electrolyzers stemming from reverse currents when power is absent. This degradation can greatly diminish the operational lifespan of the electrodes, creating a barrier to efficient hydrogen production and utilization.
Innovative Coating Approach
To combat the detrimental effects caused by reverse currents, the research team has ingeniously utilized a lead (Pb) coating on a nickel (Ni) catalyst. Traditional wisdom dictates that lead’s low activity in hydrogen evolution reactions makes it an unsuitable catalytic material. However, counteractively, the study reveals that when nickel is coated with lead, it enhances the performance of the catalyst significantly. This unconventional approach showcases how a combination of materials can yield superior results, fundamentally shifting the paradigm of catalyst design.
Lead, acting as a co-catalyst, enhances the key processes of proton desorption and water dissociation while also providing robust resistance to degradation during intermittent operation. This innovative catalyst marks a departure from previous designs that required extensive external apparatus to manage reverse current issues. With just the addition of a lead coating, this method not only boosts efficiency but also simplifies the system, providing a clearer path toward more practical hydrogen production technologies.
Transforming the Future of Hydrogen Energy
Professor Yong-Tae Kim’s remark that this is the first study to directly address reverse current degradation in AWEs using a material-based solution emphasizes the significance of this research. The implications extend beyond improved electrolysis systems; they resonate deeply within the larger context of sustainable energy transition. As the world grapples with energy demands and climate challenges, innovations like these become vital to achieving a clean, resilient energy future. The strategic integration of leading-edge materials in hydrogen production could serve as a launching pad for the next industrial revolution driven by renewable energy sources.

Leave a Reply