The profound world of organic chemistry has historically depended upon the sophisticated assembly of complex molecules, one of which has gained recent prominence: oligocyclotryptamines. As MIT chemists unveil groundbreaking methodologies to synthesize these intricate compounds, the implications for various fields, including pharmaceuticals, are profound. This article explores the innovative approaches being utilized, the significance of the compounds, and the future potential they hold for medicinal advancements.
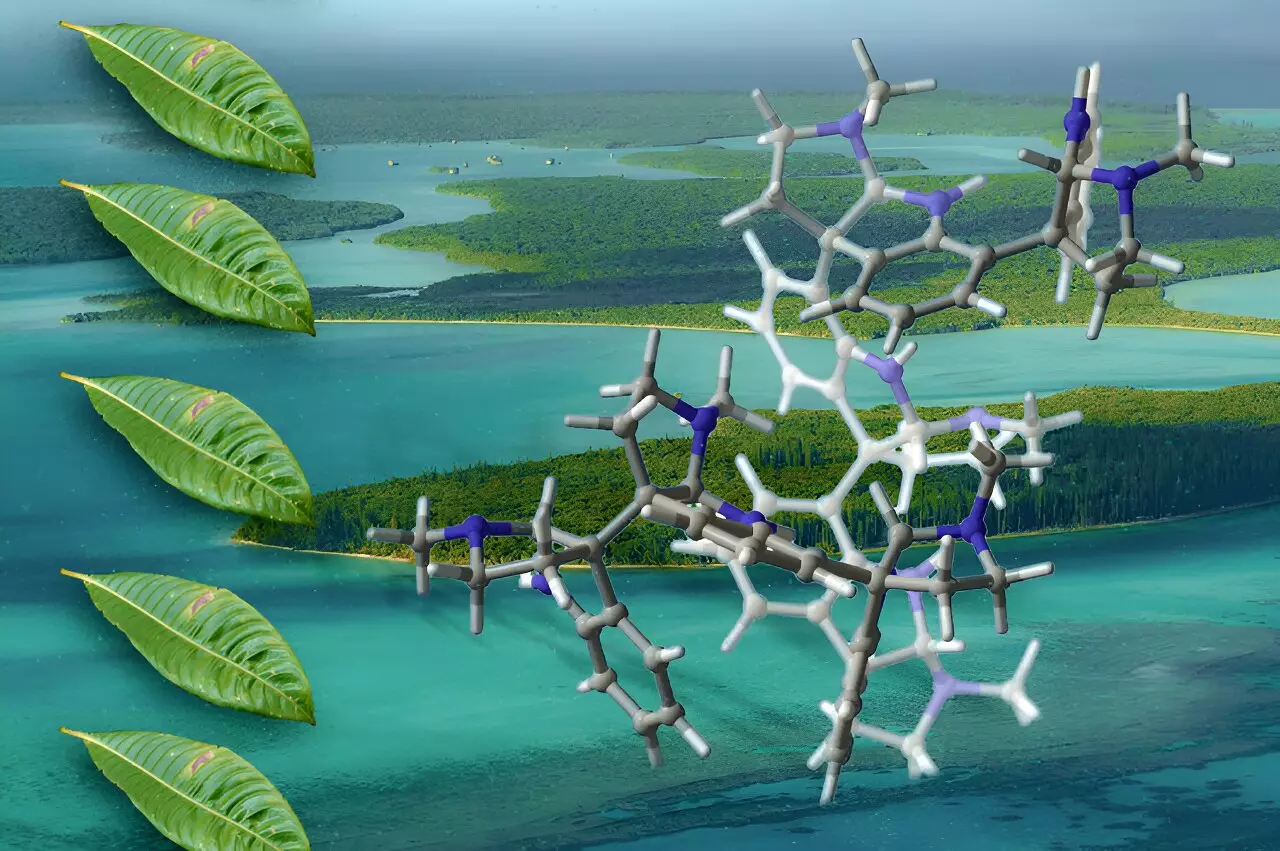
Oligocyclotryptamines are a fascinating subgroup of alkaloids, characterized by their multiple tricyclic structures known as cyclotryptamines. These molecules, primarily derived from plants like Psychotria, display a diversity that has piqued the interest of researchers across disciplines. Traditionally, the extraction of these compounds has been limited due to their scarcity in nature, which presents a significant hurdle for further pharmacological exploration. Therefore, the ability to synthesize these compounds in sufficient quantities opens new avenues for research, particularly in the development of potential antibiotics, analgesics, and cancer therapeutics.
The inherent complexity of oligocyclotryptamines arises from their multi-ring systems, wherein carbon-carbon bonds interlink the cyclotryptamine components. This interconnectedness adds a layer of difficulty in synthesizing these intricate molecules. MIT’s research team, led by Professor Mohammad Movassaghi, has spearheaded an innovative synthetic pathway that allows for the controlled assembly of these compounds. By methodically adding tryptamine-derived units to a central structure, the researchers can deftly manage the stereochemistry and conformation of each ring, effectively piecing together the molecular puzzle with precision.
The breakthrough method introduced by the MIT team leverages a diazene-directed assembly process. This technique enables two bulky carbon centers, which are typically less reactive due to steric hindrance, to be linked together efficiently. The process transforms these carbon centers into reactive radicals that can be directed to form strong bonds.
In practical terms, this synthesis starts with a base molecule and adds cyclotryptamine fragments iteratively. The selective bonding of these fragments is facilitated by breaking nitrogen bonds, creating a situation where the reactive carbon radicals quickly recombine. This method not only enhances the efficiency of the synthesis process but also ensures that the spatial orientation of the carbon atoms is maintained accurately, which is critical for the desired biological activity of the molecules.
Movassaghi’s lab had laid groundwork for this methodology in previous research focused on synthesizing different classes of alkaloids, culminating in this landmark achievement of synthesizing the largest oligocyclotryptamines yet. The work of this team not only fills a gap in existing synthetic chemistry but also exemplifies the kind of collaborative and cumulative efforts characteristic of modern scientific inquiry.
The successful synthesis of oligocyclotryptamines holds transformative potential. For decades, the therapeutic capabilities of these molecules were hindered by the insufficient quantities available for study. With reliable synthetic access, researchers can now embark on thorough investigations into the pharmacological properties of these compounds. The ability to create new variants by modifying the cyclotryptamine components offers the prospect of discovering compounds with enhanced efficacy or novel mechanisms of action.
Moreover, beyond the immediate applications, the synthesis of oligocyclotryptamines heralds a paradigm shift in organic chemistry. It offers a pathway to explore the vast arsenal of plant-derived compounds that have yet to be synthesized or studied in detail. The adaptation of this methodology extends to other families of natural products, potentially accelerating the pace of discovery in drug development.
As we stand at the forefront of this exciting research, the perspectives shared by the MIT chemists suggest that the journey is only beginning. They anticipate using this synthesized framework not merely to recreate existing compounds but to innovate and venture into uncharted territories of molecular complexity. The excitement expressed by Movassaghi and his team reflects a broader optimism in the scientific community regarding the future of natural product synthesis.
The synthesis of oligocyclotryptamines showcases the remarkable interplay between scientific theory and practical application. By overcoming challenges associated with complex biological molecules, researchers have opened doors to potential medical breakthroughs. As we continue to explore the potential of these exciting compounds, one can only imagine the transformative impacts they may soon have on medicine and beyond.
The developments at MIT exemplify a formidable leap forward in synthetic chemistry, offering hope for new therapeutic agents borne from nature’s own intricate designs. The confluence of innovation, dedication, and scientific collaboration lays a robust foundation for the future of drug discovery and development, promising to enrich our understanding of the natural world while enhancing human health.

Leave a Reply