Plastic pollution is an escalating global concern, prominently affecting marine and freshwater ecosystems. Recent studies have illuminated another layer of complexity concerning microplastics—minute plastic particles—and their behavior during seasonal freezes in aquatic environments. Research detailed in the journal *Environmental Science & Technology* provides critical insights into how ice formation and subsequent melting can alter the characteristics and environmental implications of these persistent pollutants.
Microplastics, broadly classified as plastic particles measuring less than five millimeters, originate from various sources, including the breakdown of larger plastic debris and the shedding of synthetic fibers from textiles. Once they enter aquatic systems, their presence raises significant ecological concerns. As the latest research indicates, the behavior of microplastics in water bodies is not static; rather, it can be profoundly affected by environmental factors such as temperature, salinity, and notably, the freezing of water.
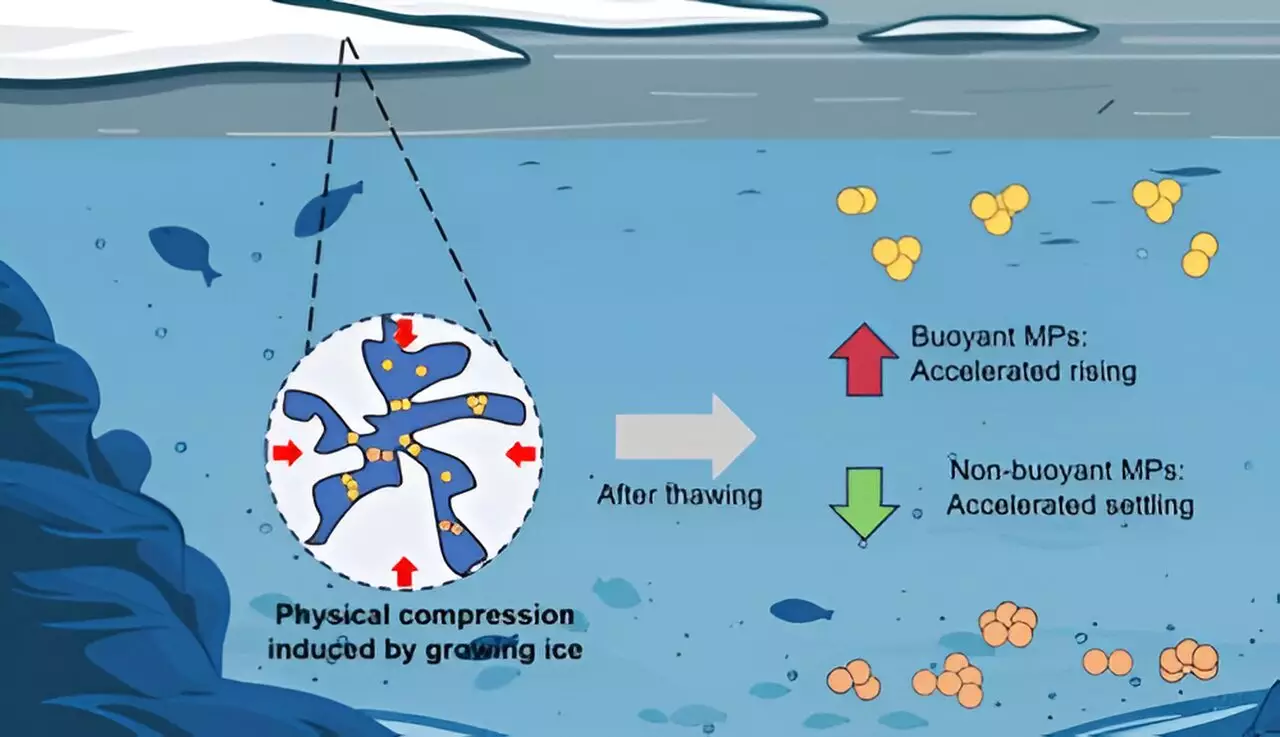
The study led by Chunjiang An and colleagues at a research institution probes the transformation of microplastic behavior during cycles of freezing and thawing. It highlights that these particles undergo physical changes when subjected to ice, with implications that could have far-reaching effects on sediment accumulation processes in freshwater systems and beyond.
The Experiment: Unveiling Particle Transformation
To investigate the effects of freezing on microplastic particles, the researchers selected three common polymers frequently encountered in microplastics: polyethylene (PE), polyurethanes (PU), and polytetrafluorethylene (PTFE). These polymers exhibit distinctive properties, influencing their buoyancy and, consequently, their behavior in aquatic environments. The team experimentally subjected particles of these polymers to a controlled freezing process for 24 hours in both freshwater and saline solutions.
The findings from these experiments revealed that the size of particles increased post-freezing compared to a control group held at cooler temperatures. Notably, PE particles demonstrated the most substantial growth—46% larger—while PU particles only increased by 9%. The authors theorize that PE’s hydrophobic nature contributed to its reduced dispersion capability compared to PU. Understanding these particles’ characteristics aids in grasping how they may interact chemically with their environment, posing different threats depending on their size and density.
Why Does Buoyancy Matter?
The implications of buoyancy cannot be overstated in assessing the fate of microplastics in aquatic systems. Floating particles are more likely to remain suspended in the water column, potentially leading to ingestion by marine organisms. In contrast, sinking particles can contribute to sedimentation in lake and riverbeds—altering the sediment’s chemical composition and potentially disrupting local ecosystems.
The research further explores that as salinity levels increased, the effect of freezing on the polymers’ sizes diminished, possibly due to the formation of brine channels in the ice that prevented particle clumping. This nuanced interaction between salinity and microplastic behavior is crucial in understanding how these pollutants might behave differently across various aquatic environments, especially as climate change alters salinity and temperature patterns globally.
Understanding the dynamics of microplastics, especially in cold regions where ice covers water bodies for extended periods, offers essential insights into their long-term environmental impact. The altered buoyancy and size post-freezing suggest a potential increase in the accumulation of these plastics in sediments once the ice melts. Consequently, this could lead to a higher concentration of microplastics in the food web, fostering bioaccumulation in aquatic organisms and making it imperative to consider seasonal cycles in pollution studies.
Despite the findings, the research acknowledges the limitations of the experimental conditions, citing that natural freeze-thaw cycles are more prolonged. Therefore, further investigations are crucial to comprehensively understand the effects of extended freezing periods on microplastics’ ecological fates.
As the growing body of knowledge regarding microplastics unveils the nuanced interactions between these particles and their environments, this study serves as a crucial stepping stone in the field. With a clearer picture of how seasonal ice impacts microplastic behavior, researchers, policymakers, and the public must work collaboratively to address the pervasive issue of plastic pollution, advocating for sustainable practices to mitigate its future prevalence in ecosystems around the globe.

Leave a Reply