Recent advancements at the Fritz Haber Institute, Sorbonne University, and Uppsala University have unveiled pivotal findings in the study of ions in solutions. This collaborative effort, encapsulated in their paper titled “The solvation shell probed by resonant intermolecular Coulombic decay,” published in Nature Communications, opens new avenues for understanding the intricate interactions between ions and their solvent environments. As scientists strive to elucidate these interactions, their work promises to enhance our grasp of fundamental chemical processes.
At the heart of this research lies the concept of solvation shells, layers of solvent molecules that form around dissolved ions or molecules when they encounter a liquid medium. These shells are not mere passive entities; they possess unique properties that can significantly diverge from those of the surrounding solvent. Traditional methods for studying these structures have been hampered by their complexity and the challenge of isolating the solvation shells from the plethora of solvent molecules in solution. Consequently, there exists a pressing need for innovative techniques capable of shedding light on the behavior of these elusive shells.
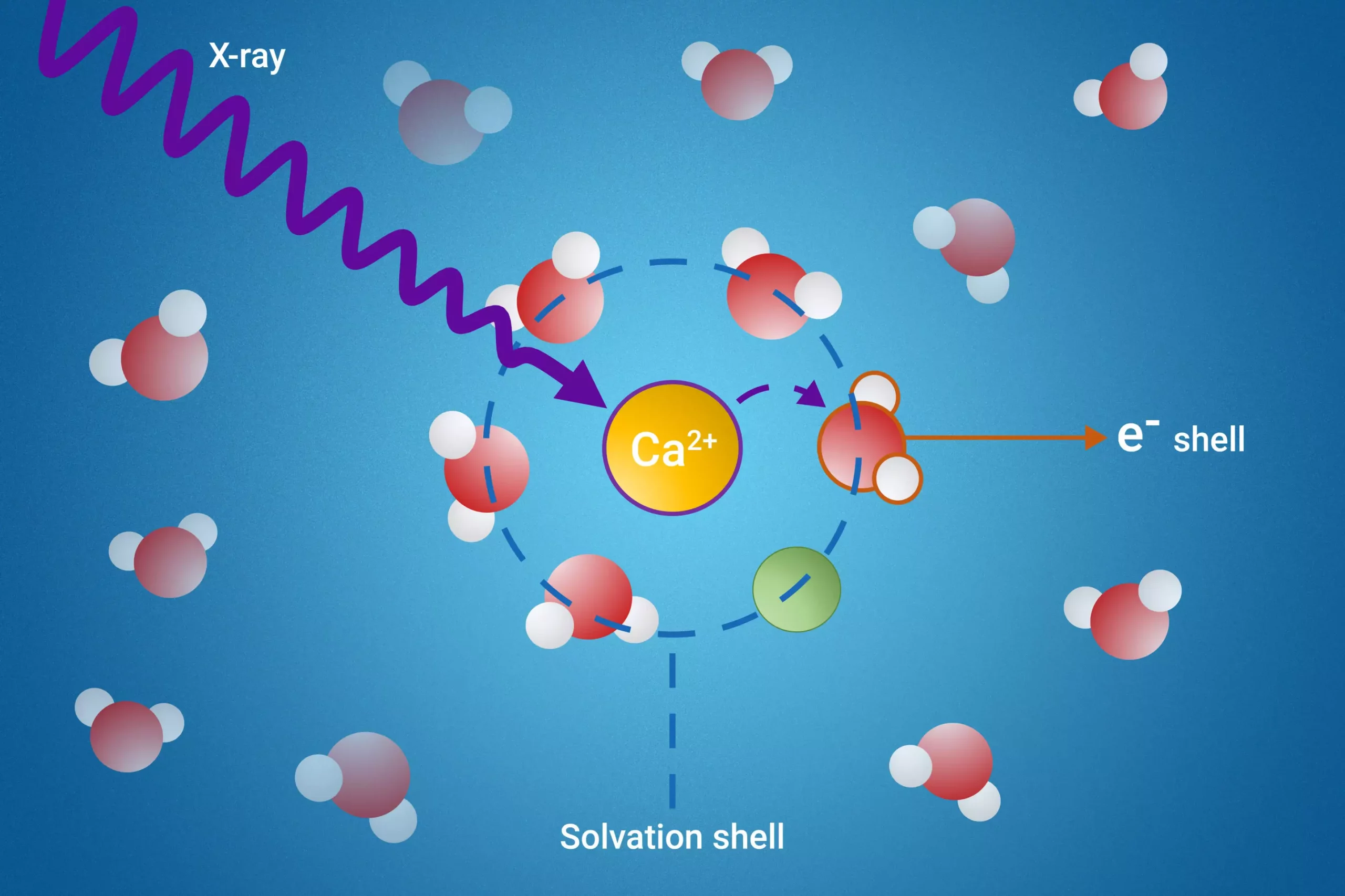
The research team introduced a groundbreaking method utilizing resonant intermolecular Coulombic decay (ICD), a process that involves the excitation of molecules through X-ray irradiation. This excitation triggers a decay process that reveals vital information about the interactions occurring within the solvation shell. By scrutinizing how excited molecules interact with their neighboring solvent molecules during the decay, the researchers have created a lens through which the properties of solvation shells can be analyzed with unprecedented clarity.
The significance of this method extends beyond mere observation; it serves as an indicator for ion pair formation and allows for the measurement of electron binding energies specific to the water molecules within the first solvation shell—an achievement previously thought impossible. These insights are crucial, as they provide a deeper understanding of how solvent structures influence chemical reactivity and stability.
The Broader Implications of Solvation Studies
The implications of this research reverberate across various scientific disciplines. The nuances of solvation are foundational to fields such as chemistry, biology, materials science, atmospheric science, and electrochemistry. Improved comprehension of solvation dynamics can lead to innovations in drug design, environmentally friendly materials, and energy storage solutions. Moreover, this exploration of solvation shells plays a pivotal role in developing sustainable technologies that depend on precise chemical interactions.
The work conducted by this international team represents a crucial step forward in understanding ion behavior in solutions through the examination of solvation shells. Their novel approach employing resonant intermolecular Coulombic decay not only reveals the complexities of molecular interactions but also establishes a valuable tool for future studies. As scientists continue to peel away the layers surrounding these solvation phenomena, we can anticipate transformative advancements in both theoretical frameworks and practical applications across numerous scientific and engineering domains.

Leave a Reply