The quest to mitigate climate change has propelled scientists toward innovative approaches to carbon dioxide (CO2) reduction. Capturing and converting CO2 emissions into valuable resources is not only essential for sustainability but also pivotal for establishing a circular economy. Recent breakthroughs in understanding the nuances of CO2 conversion reactions have opened doors to enhancing the efficiency and selectivity of these processes. A significant advancement comes from a study conducted by a team of researchers at the University of Twente, highlighting the intricate relationship between the chemical environment and the reduction of CO2 on copper electrodes.
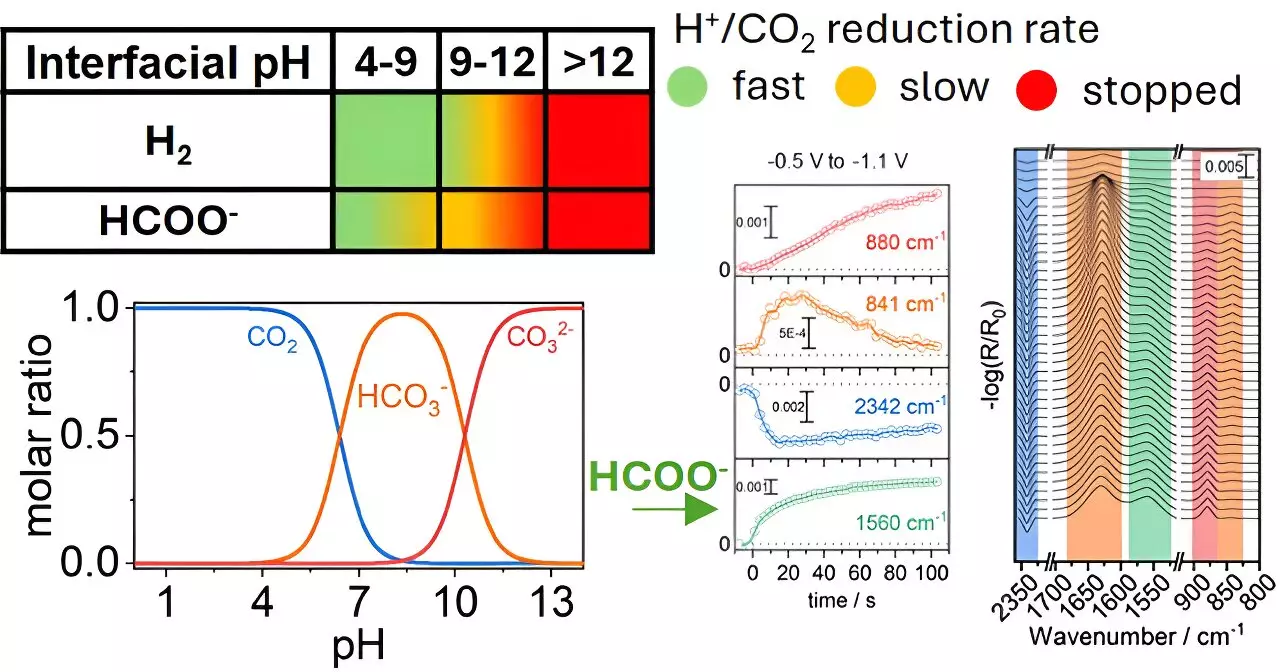
At the core of the research conducted by Georgios Katsoukis and his team lies the realization that the interactions on the surface of copper electrodes are profoundly affected by the local chemical environment, particularly the pH levels. Traditional methods have often focused solely on improving catalyst materials. However, this study illustrates that optimizing the surrounding conditions can dramatically enhance the conversion rate of CO2 into formate, an essential compound with numerous industrial applications. By manipulating the pH levels near the copper electrodes, the researchers identified that these parameters significantly influence not only the rate of reaction but also the selectivity of the products formed.
One of the longstanding hurdles in CO2 reduction technology is achieving selectivity, as various reaction products can emerge under different conditions. This complexity complicates the development of efficient systems for CO2 conversion. The findings from the University of Twente team challenge the reductionist view that merely enhancing catalyst materials will yield better results. Instead, it underscores the necessity of a holistic approach that considers the intricacies of the chemical environment as a key factor in determining reaction pathways.
The implications of this research extend beyond immediate applications. The insights gathered provide a valuable blueprint for future studies aimed at refining CO2 reduction technologies. By focusing not only on the catalysts but also on fine-tuning the chemical environments during the reactions, researchers can push the boundaries of efficiency and selectivity. This dual approach could enhance not only the conversion efficacy but also prolong the functional lifespan of the electrodes, addressing a critical aspect of system durability.
The exploration of the interplay between chemical environments and CO2 reduction on copper electrodes marks a significant milestone in the field of catalysis. By emphasizing the importance of these conditions, researchers are providing insights that could reshape the development of carbon reduction technologies. As we strive towards sustainable solutions for ecological challenges, this approach brings us closer to realizing effective methods for transforming CO2 emissions into useful resources, ultimately fostering a more sustainable and circular economy. The pathway is now clearer for scientists and innovators as they embark on the journey to optimize not just catalysts but the very environments in which these crucial reactions take place.

Leave a Reply