In the ever-evolving field of chemical engineering, researchers are exploring innovative methods to enhance the efficiency of chemical separation processes. A recent breakthrough from a team at the University of Illinois Urbana-Champaign introduces a specialized polymer that selectively attracts certain substances only when electrically activated. This development presents a promising shift towards more sustainable and efficient methods of chemical separation, which is particularly relevant in industries such as pharmaceuticals and chemical synthesis.
What sets this polymer apart is its functionality grounded in electrochemistry. Traditional methods of chemical separation, such as heat-based processes or membrane filtration, often generate substantial material waste and lack specificity in the substances they target. The new polymer utilizes halogen bonding to create a selective interaction, thereby maximizing the efficacy of chemical separation. As explained by Professor Xiao Su, the lead researcher, this polymer functions similarly to a “sponge” designed to absorb only the desired chemicals from a diverse mixture.
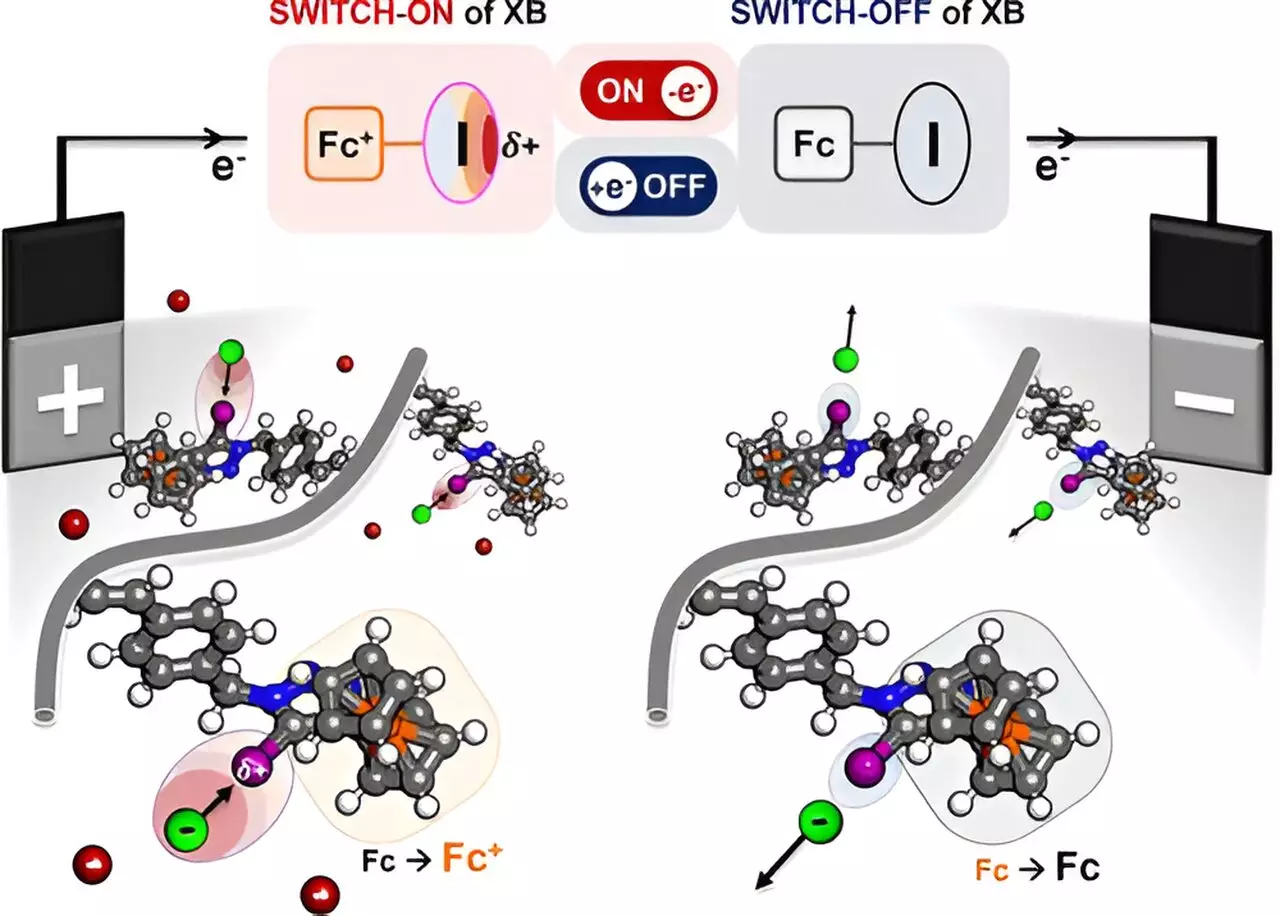
The innovation lies in the polymer’s ability to manipulate the charge density of a halogen atom, specifically iodine, under electrical activation. When electricity is applied, the polymer modifies the bonding characteristics of iodine, generating a strong positive concentration that attracts negatively charged entities like halides and oxyanions. This “electric sponge” concept is revolutionary, as it allows for precision in targeting specific ions and molecules.
Halogen Bonding: The Key to Selectivity
Central to this advancement is the concept of halogen bonding, a phenomenon characterized by the attraction between a positively charged halogen atom and negatively charged ions. By leveraging this interaction, Su’s research team has successfully crafted a redox-active polymer that exemplifies the principle of selective attraction. The study outlines the transformation of the iodine atom’s charge state when ferrocene—a highly active redox center—undergoes oxidation.
This selective mechanism takes advantage of the unique properties of halogen atoms, particularly their ability to create partial positive charges. Such precision in targeting molecules from organic solutions could reshape the landscape of chemical processing, leading to decreased waste and enhanced purity of products. The initial findings, confirmed through rigorous testing, indicate that specific ions are indeed isolated from complex mixtures, showcasing the practical viability of this method.
The implications of this technology in industrial applications are profound. As industries face increasing pressure to minimize environmental impact, traditional methods that contribute to waste and energy consumption need reevaluation. The polymer’s reliance on electrochemical mechanisms can significantly reduce waste and leverage renewable energy sources, aligning with global sustainability goals.
The potential to adapt this polymer for large-scale applications remains a priority for the research group. Future efforts will focus on refining and scaling the process, which involves devising innovative strategies such as a cascade model to enhance final product purity. Additionally, the team aims to create a continuous electrosorption system that could operate effectively outside controlled laboratory conditions.
The groundbreaking work conducted at the University of Illinois Urbana-Champaign heralds a new approach to chemical separation that could dramatically improve the efficiency and sustainability of industrial processes. Through electrochemical mechanisms and the selective attraction of target molecules via halogen bonding, this polymer represents a transformative leap in technology. As research progresses toward refining and scaling these processes, we stand on the cusp of a new era in chemical engineering—a future wherein efficient and waste-conscious chemical separation becomes a standard practice in various industries. The continued exploration of these innovative materials could pave the way for solving several contemporary challenges, ultimately benefiting both the environment and industrial efficiency.

Leave a Reply