Humanity has long pondered the profound questions of existence: Who are we, and why are we here? These existential musings echo the lyrics of Crosby, Stills, Nash & Young’s classic song, reminding us that our very being can be traced back to the stardust formed in the depths of space. Scientific inquiry into these cosmic roots has intensified, as researchers seek to unravel the mysteries behind the formation of prebiotic molecules—essential building blocks for life, both on Earth and potentially beyond. Through innovative experiments, especially those involving extraterrestrial chemical processes, the scientific community is inching closer to understanding the conditions conducive to life’s emergence in the universe.
Recent research has highlighted the critical role that low-energy electrons play in the synthesis of these vital prebiotic molecules. Groundbreaking experiments conducted by a team led by undergraduate student Kennedy Barnes at Wellesley College, along with mentorship from esteemed faculty members, have unveiled insights into how cosmic radiation interacts with various materials, particularly ice, present in outer space. Their work suggests that low-energy electrons generated by cosmic rays may hold greater significance in chemical reactions when compared to photons, the traditional focus of earlier studies.
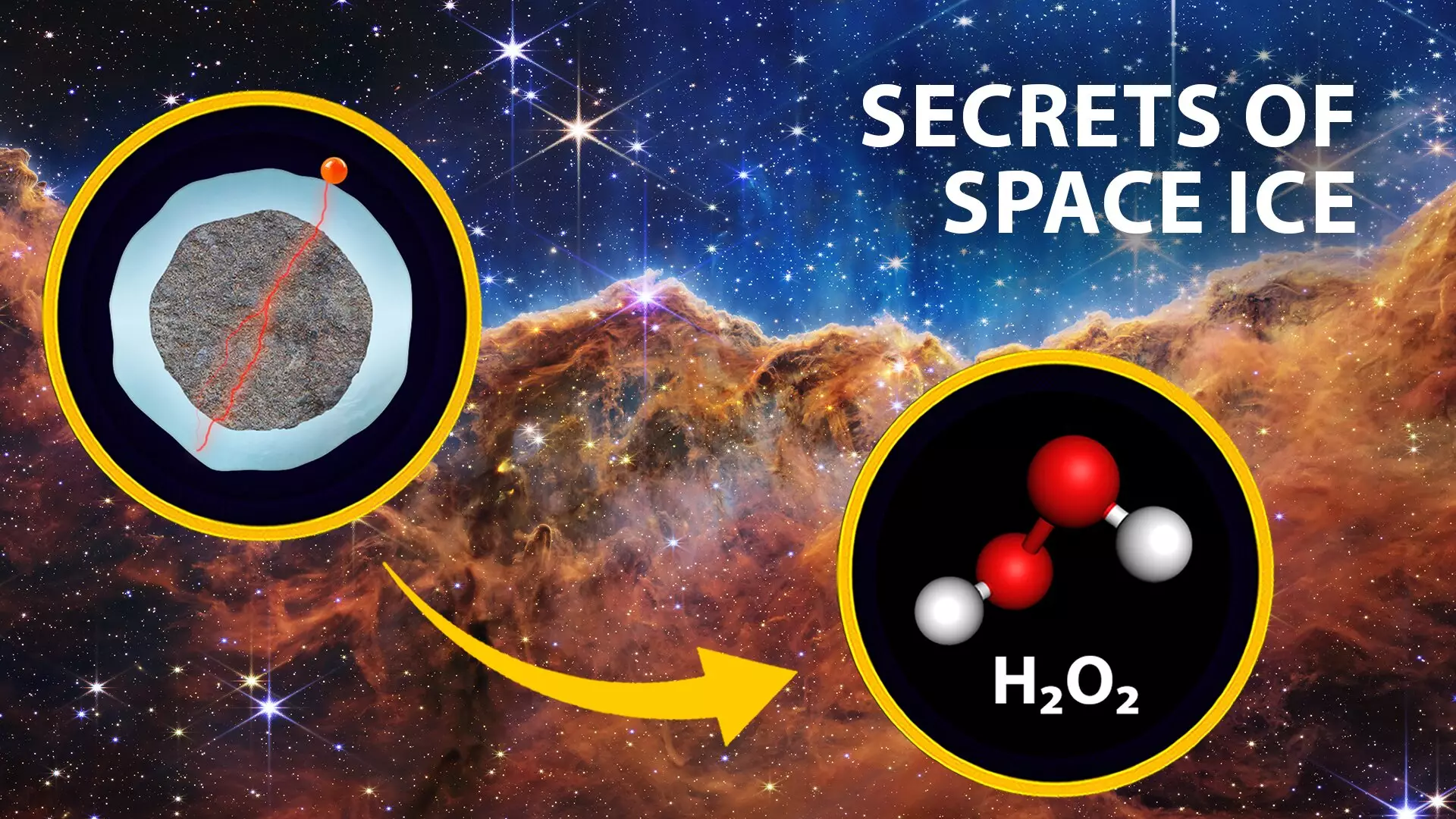
This shift in perspective arises from calculations indicating that cosmic-ray-induced electrons may outnumber photons significantly within cosmic ice environments. As Barnes articulated, “The number of cosmic-ray-induced electrons could be much greater than the number of photons striking the ice.” This revelation hints at a profound understanding of how these particles contribute to the synthesis of prebiotic molecules under extraterrestrial conditions, paving the way for exciting discussions in astrochemistry.
While the implications of this discovery resonate across cosmic distances, they also extend to pressing concerns on our home planet. The research team’s investigation into low-energy electrons does not conclude with space chemistry; it also encompasses practical applications on Earth. By exploring the radiolysis process in water, the team uncovered evidence that these electrons can stimulate the production of harmful byproducts such as hydrogen peroxide and hydroperoxyl radicals. These molecules, in turn, have implications for both environmental and medical concerns, influencing ozone depletion and impacting cellular health.
For instance, Barnes emphasized the potential of their findings in medical practices, particularly in cancer treatment modalities. Here, the concept of employing high-energy radiation aligns with existing methodologies where water’s composition interacts with radiation, affecting DNA integrity. Such knowledge can help optimize therapeutic approaches, making strides toward a more nuanced understanding of the cellular effects of radiation.
An integral aspect of the team’s approach is their commitment to replicating the conditions of space within laboratory settings. Using cutting-edge technology—including an ultrahigh-vacuum chamber, an electron gun, and a laser-driven plasma lamp—researchers have ingeniously created controlled environments where ice films are bombarded with electrons and photons. This experimental framework allows scientists to observe the resultant chemical reactions and identify the specific molecules formed, further corroborating their hypothesis regarding the superior effectiveness of low-energy electrons.
In addition to studying submicron ice particles akin to those found in space, the findings will deepen our understanding of larger cosmic ice bodies, such as Europa, Jupiter’s intriguing moon encased in a thick layer of ice. Research outcomes may serve as key data points for upcoming space missions, including those spearheaded by NASA, which aim to capture and analyze the conditions on such distant celestial bodies.
As the Barnes-led team continues to delve into the intricacies of molecular composition within ice films and broader prebiotic chemistry, their work invites fellow researchers to consider incorporating low-energy electrons in models that simulate extraterrestrial environments. Collaborations with international partners in France further enrich this endeavor, as they explore various atomic reactions and chemical pathways.
In an era rife with discoveries, the prospect of understanding life’s origins becomes tantalizingly close. “There’s a lot that we’re on the cusp of learning,” expressed Barnes enthusiastically. Such statements signal an exhilarating phase in scientific exploration—one where the study of astrochemistry not only elucidates our beginnings but also enhances our present-day capabilities and environmental stewardship. By bridging cosmic research with terrestrial applications, this branch of study inspires a holistic understanding of our universe and our place within it.

Leave a Reply