Organic redox-active molecules (ORAMs) have emerged as promising candidates in the realm of energy storage solutions, particularly within the growing field of aqueous organic flow batteries (AOFBs). Their diverse nature presents an exciting opportunity to develop cost-effective and sustainable energy systems. However, the practical application of ORAMs has been hindered by challenges such as instability during the charge-discharge cycles and susceptibility to side reactions that can deactivate their redox activity. This instability raises concerns about the longevity and effectiveness of AOFBs when using these organic compounds.
One critical factor in the deployment of ORAMs is air stability. Many of these molecules are reactive and can degrade when exposed to atmospheric conditions, which poses serious limitations for their effective utilization in real-world applications. Researchers face the daunting task of creating ORAMs that not only possess robust electrochemical properties but can also withstand atmospheric exposure without losing functionality. This necessity has driven recent research towards the development of more stable chemical frameworks.
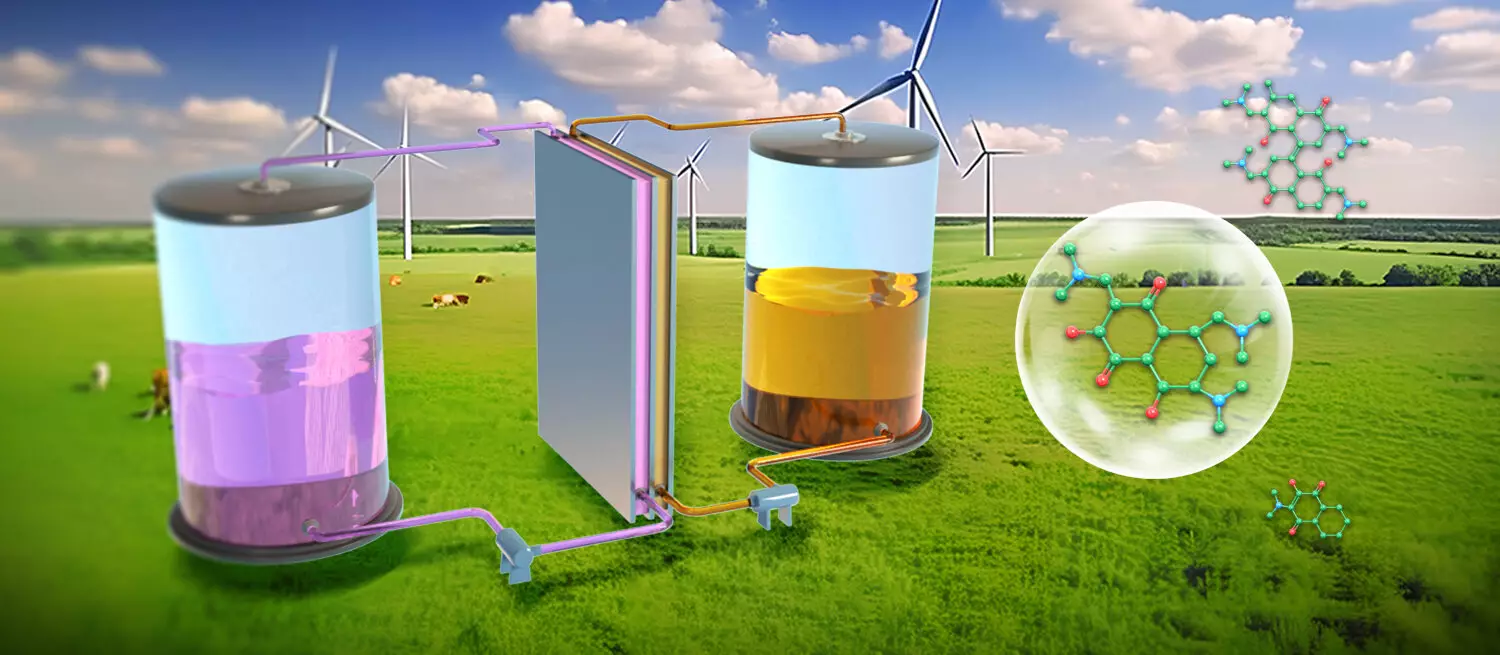
A recent breakthrough from the Dalian Institute of Chemical Physics underscores the evolving landscape of ORAM research. Led by Professors Li Xianfeng and Zhang Changkun, the team introduced novel naphthalene derivatives infused with active hydroxyls and dimethylamine scaffolds. These new compounds exhibit impressive air stability and serve as effective catholytes in AOFBs. The findings, published in *Nature Sustainability*, suggest that these newly engineered ORAMs not only enhance performance but also present a promising solution to the prevalent issue of degradation during battery cycling.
The researchers utilized a scalable synthetic method that integrated chemical strategies with in situ electrochemical techniques, greatly simplifying the purification process and reducing production costs. This innovative approach facilitated the creation of multisubstituted naphthalene derivatives, which demonstrate enhanced solubility in aqueous electrolytes and protective characteristics against side reactions. Through exhaustive testing, the resulting AOFB demonstrated an impressive capacity retention over numerous cycles, showcasing the practical potential of these derivatives in energy storage applications.
The naphthalene-based AOFB successfully achieved stable cycling for about 850 cycles over 40 days, maintaining a capacity of 50 Ah L-1. Even with continuous air exposure in the catholyte, remarkable performance was sustained for around 600 cycles, equivalent to roughly 22 days, without declines in capacity or efficiency. Furthermore, the research team successfully scaled the synthesis to a remarkable 5 kg per batch, confirming the feasibility of producing these derivatives at a commercial scale. The pilot-scale testing showed an average system capacity of approximately 330 Ah, with an exceptional capacity retention rate of 99.95% across 270 cycles.
The pioneering work led by Prof. Li and his team promises not only to advance the capabilities of AOFBs but also to pave the way for future innovations in sustainable energy storage technologies. By addressing the critical challenges of air stability and synthesis efficiency, this research marks a pivotal moment in the field of electrochemical energy storage. As the world seeks greener energy solutions, the development of stable and cost-effective ORAMs like the naphthalene derivatives will play a crucial role in the evolution of sustainable energy systems.

Leave a Reply