The immunoproteasome plays a crucial role in the cellular immune response, acting as a key player in the identification and breakdown of foreign invaders such as bacteria and viruses. This specialized enzyme complex dismantles intruders into manageable fragments that can be presented to immune cells, effectively educating the immune system on how to target these threats. This process not only enhances immune efficiency but is vital for initiating adaptive immune responses. However, when the immunoproteasome becomes overactive, it can misidentify the body’s own structures as foreign, leading to autoimmune diseases. Finding a balance in immunoproteasome activity is essential for maintaining immune health.
For years, researchers have grappled with the challenge of selectively inhibiting the immunoproteasome. The complexity lies in the necessity to spare other proteasome variants that are vital for cellular functions such as recycling and waste disposal. This selectivity is crucial because non-selective inhibition can lead to detrimental side effects, exacerbating rather than alleviating the very conditions researchers aim to treat. The task has been complicated by the overlapping functions of different proteasomal complexes. Considering the risk of side effects, it is imperative that any new drugs maintain a high degree of specificity when targeting the immunoproteasome.
A Breakthrough in Drug Design
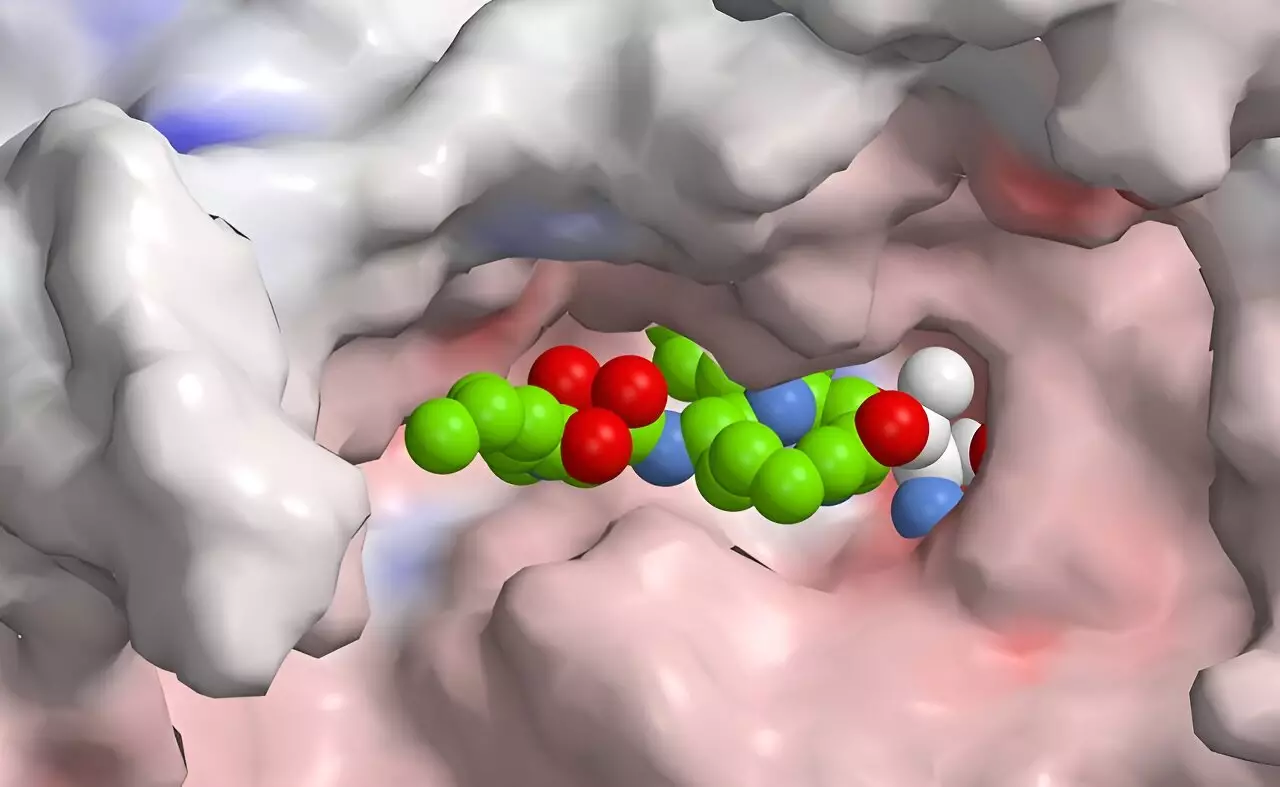
Researchers led by Helge Bode at the Max Planck Institute have made significant strides in this arena by developing a novel approach to produce selective inhibitors. They manipulated the biosynthesis of natural substances derived from bacteria, which has led to the creation of a new class of drugs with the potential to selectively inhibit the immunoproteasome. Working alongside esteemed colleagues from institutions such as the Technical University of Munich and the University of Duisburg-Essen, the team has synthesized a hybrid compound that merges peptides with polyketides. This pioneering work stands at the intersection of synthetic biology and medicinal chemistry.
The innovative method they utilized involved the XUT technology, which exploits specific docking sites within thiolation domains accessible in both non-ribosomal peptide synthetases and polyketide synthases. This fusion suggests a biological precedent where nature itself combines these two pathways to create hybrid structures, similar to syrbactins, which are known to target and damage proteasome systems in a variety of higher organisms.
Insights from Nature’s Design
Syrbactins are intriguing bioactive compounds produced by certain bacteria, known for their ability to inhibit proteasome activity. This inhibition can lead to cellular death, a property that has garnered interest for potential cancer therapeutic applications. By understanding and modifying the structure of these natural hybrids, Bode’s team aims to generate molecules with better selectivity toward the human immunoproteasome, thus minimizing harmful side effects common with existing proteasome inhibitors. Through rational design and iterative modifications, the researchers believe that the future holds the promise of refined compounds that could treat autoimmune diseases effectively and with fewer complications.
While the current hybrid compound developed by Bode and his team is yet to achieve optimal selectivity, it represents a significant step forward. Their work not only opens avenues for targeted immunoproteasome inhibitors but also sets the stage for future innovations. The potential of computer-assisted drug design combined with high-throughput screening will enable researchers to rapidly identify and develop improved variants. This methodological evolution could redefine the landscape of treatment options for autoimmune diseases, signaling a shift toward more personalized and precise medical interventions.
The convergence of synthetic biology and medicinal chemistry in addressing the challenges of selective immunoproteasome inhibition heralds a promising future for the treatment of autoimmune disorders. With ongoing research and development, there is hope for new therapies that not only target the underlying causes of these diseases but do so with greater efficacy and fewer side effects than current options. As this field progresses, it could lead to transformative approaches that ultimately improve patient outcomes and advance our understanding of the immune system’s complexities.

Leave a Reply