Water is a vital resource for life on Earth, yet contamination from heavy metals poses a significant threat to ecosystems and human health alike. Metals such as cadmium and lead, often found in mining runoff, industrial discharge, and agricultural practices, have been linked to various health issues, including neurological damage and cancer, even at low concentrations. As urban areas expand and industrial activities increase, the risk of heavy metal pollution in drinking water and natural water bodies becomes critical. Traditional purification methods, including extensive filtration systems, often prove inadequate and resource-intensive, necessitating the exploration of alternative solutions.
Recent advancements in biomimicry and polymer science have opened new avenues for water treatment, leading scientists to investigate plant-derived materials for their natural filtering capabilities. Plants, through their complex polysaccharides, form protective barriers that can absorb and immobilize harmful metal ions in their cellular structure. However, many of these natural polymers face challenges regarding solubility and stability, requiring additional chemical agents for effective use in water treatment applications.
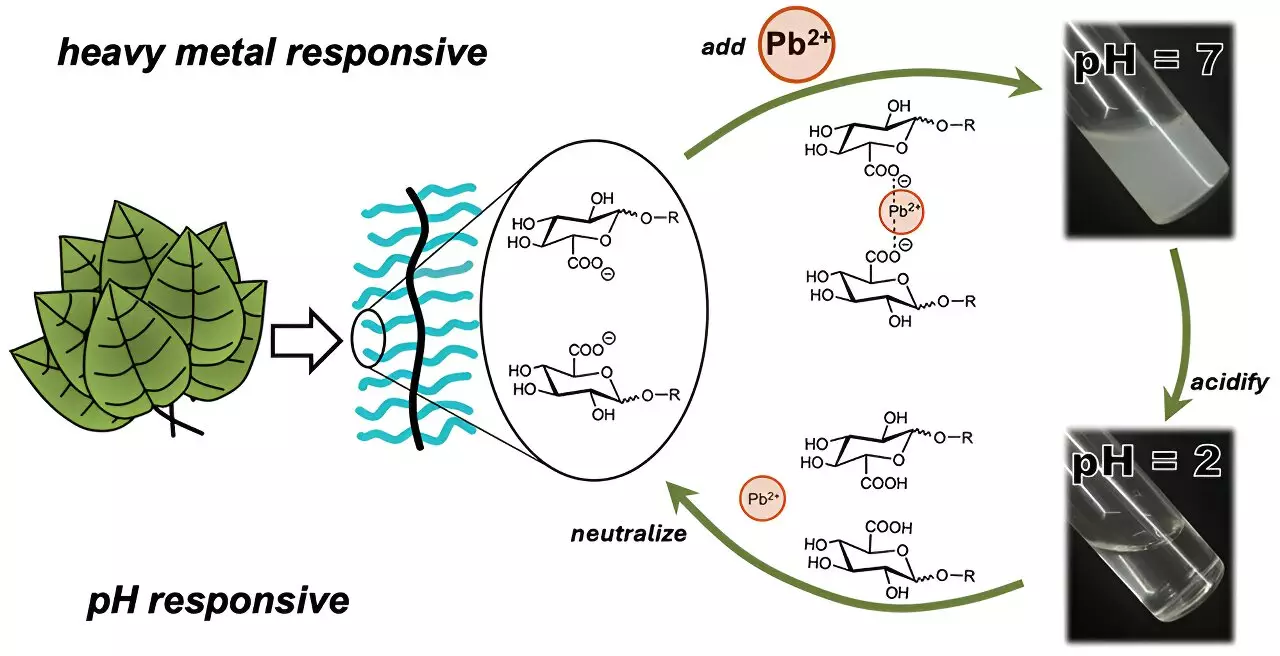
This exploration has led researchers to investigate the potential of creating engineered polymers that can effectively bind heavy metals while minimizing reliance on external additives. A promising development has emerged from the University of Texas at Austin, where a research team has synthesized a unique sugar-like polymer designed to capture heavy metals in a more efficient and environmentally friendly manner.
Cassandra Callmann and her team focused on developing a single polymer capable of removing heavy metals without the drawbacks associated with traditional methods. Their innovative approach involved constructing a polymer with an insoluble backbone structure, akin to a framework, adorned with water-soluble carbohydrate “charms” that interact with heavy metal ions. Among the various designs tested, the carbohydrate containing a carboxylic acid group showed remarkable efficiency in binding ionic cadmium.
In practical tests, this polymer exhibited its effectiveness within three minutes of contact with spiked water samples. It produced visible clumps that could be readily filtered, unlike many existing solutions that require complex processes and equipment to remove contaminants. This rapid action highlights the polymer’s potential for real-world applications, where swift responses to contamination are crucial.
One of the significant advantages of this newly developed polymer is its reusability. After undergoing cycles of binding and removing cadmium from the water, the polymer has shown the ability to redissolve by simply adjusting the water’s acidity. This feature not only enhances its practicality but also aligns with sustainability goals by reducing waste and the need for frequent replacements of purification materials. The researchers observed that even after three cycles, the polymer maintained its efficiency in removing heavy metals, indicating a considerable leap forward in the development of reusable water purification technologies.
The team did not stop with preliminary tests; they took their research a step further by evaluating the polymer in real-world conditions. By introducing the polymer to spiked Colorado River water, they assessed its ability to capture cadmium and lead amid a cocktail of other ions like calcium, sodium, and magnesium present in natural water sources. The results were promising: the polymer efficiently captured significant percentages of the targeted heavy metals while minimal amounts of the benign ions were affected. This balance demonstrates the polymer’s selectivity, which is crucial for future applications to avoid unintentionally removing essential minerals from drinking water.
Though the results are encouraging, further research and real-world testing are imperative to fully understand the potential implications of this technology. Issues such as long-term performance, the impact of varying environmental conditions, and cost-effectiveness will need to be addressed before widespread implementation. However, this breakthrough represents significant progress in the quest for more efficient, sustainable, and safe water purification methods to combat heavy metal pollution, as it not only simplifies the overall process but also offers a viable alternative to current strategies.
With ongoing advancements and increased collaboration between researchers and industry, the hope is that such innovations can ultimately lead to cleaner, safer water resources for communities around the world.

Leave a Reply