In recent years, the urgency to mitigate harmful industrial emissions has reached critical levels. Among the most pressing pollutants are nitrogen oxides (NO and N2O), notorious for their adverse effects on human health and the environment. These gases are produced primarily during several industrial processes, including fertilizer manufacturing. Hence, finding effective ways to minimize their release has become an essential challenge for both researchers and industries alike. In this context, zeolite-based catalysts have emerged as pivotal tools for combatting nitrogen oxide emissions, enhancing our ability to not only understand these compounds but also to innovate solutions that address this environmental crisis.
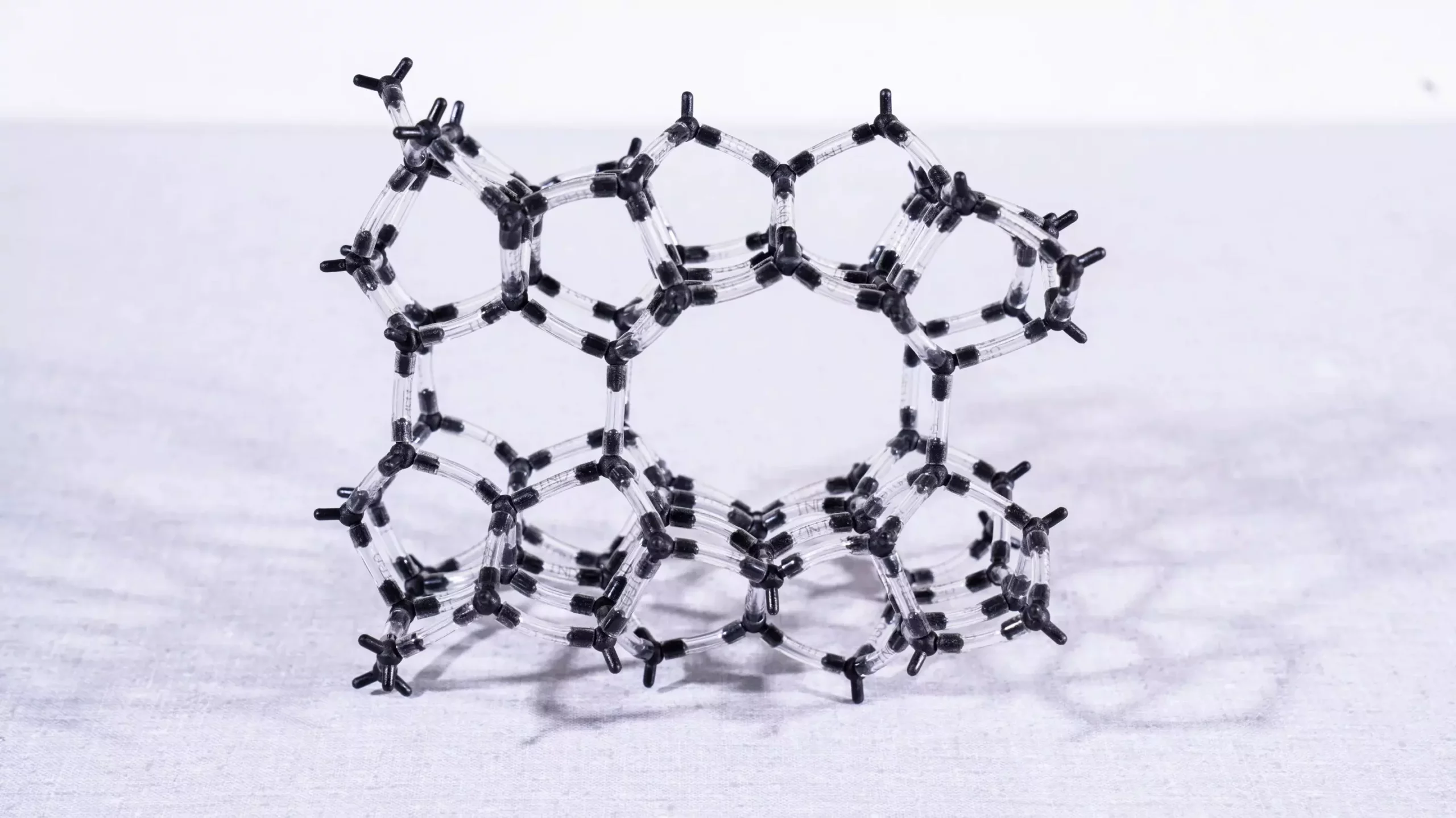
Zeolites, with their unique nano-porous framework composed mainly of aluminum, silicon, and oxygen, exhibit exceptional catalytic properties. These naturally occurring or synthetically produced minerals facilitate various chemical reactions due to their tunable structures and the ability to incorporate different active elements, such as iron. The iron within the zeolite matrix plays a crucial role, as its specific positioning and interactions are vital for effective catalysis. Recent studies conducted by researchers at the Paul Scherrer Institute (PSI) and CASALE SA have shed light on how various iron species within zeolites interact to drive reactions that convert toxic nitrogen oxides into harmless substances.
The Discovery of Iron Species Interaction
The intriguing finding from the PSI research is that individual iron atoms located in neighboring pores communicate with each other, catalyzing the desired chemical transformations in a concerted manner. Filippo Buttignol, a principal researcher involved in the study, emphasizes that the presence of iron atoms varies widely in size, position, and bonding configuration within the zeolite framework. This variety creates numerous iron species—ranging from single atoms to complex multi-atom clusters—that collectively contribute to the catalytic process. Understanding which specific iron species are responsible for removing nitrogen oxides is crucial for optimizing catalyst design.
Innovative Spectroscopic Techniques Unveil Mechanisms
To unravel the complexities of iron species interactions during nitrogen oxide conversion, the researchers employed three cutting-edge spectroscopic techniques, revealing a comprehensive picture of the catalytic process. X-ray absorption spectroscopy enabled the simultaneous examination of all iron species present, while electron paramagnetic resonance spectroscopy provided insights into the individual contributions of each species. Finally, infrared spectroscopy helped to delineate the molecular dynamics at play.
The culmination of these investigations highlighted a precise arrangement of iron atoms in the zeolite structure. Specifically, it demonstrated that catalysis occurs at two particular iron sites that work together to expedite the conversion of N2O and NO. This aligned configuration allows both iron atoms to participate in essential redox reactions, continuously cycling electrons to facilitate the breakdown of these harmful gases.
The implications of this research extend far beyond basic scientific inquiry. The nuanced understanding of catalysis mechanics can drive innovations in catalyst manufacturing. By pinpointing where effective chemical reactions take place within the zeolite framework, manufacturers can tailor the synthesis of these catalysts for maximum efficiency in nitrogen oxide abatement. As Davide Ferri, head of the Applied Catalysis and Spectroscopy research group at PSI, notes, identifying specific reaction sites informs the design of improved catalysts that can better combat industrial emissions.
Broader Environmental Impact
Removing nitrogen oxides from industrial emissions is not only a matter of public health but also a critical factor in environmental sustainability. Nitric oxide contributes significantly to acid rain formation, while nitrous oxide has up to 300 times the greenhouse gas effect of carbon dioxide. The findings from PSI represent a significant step in advancing techniques that can mitigate these impacts. By better understanding the roles of zeolite catalysts, we can develop more potent solutions for air quality management and climate change, ultimately fostering a healthier planet.
Zeolite-based catalysts play an indispensable role in reducing harmful nitrogen oxides from industrial emissions. The synergy between individual iron atoms within these complex structures is at the core of these reactions, offering a pathway to more effective catalytic solutions. Continued research in this field promises not only to enhance our understanding of these mechanisms but also to pave the way for cleaner, safer industrial practices that prioritize environmental health. Embracing these advancements is essential as we strive to tackle the pressing challenges of air quality and climate change in an increasingly industrial world.

Leave a Reply