The realm of polymer chemistry has long been captivated by the complex behavior of polyelectrolytes—charged polymers that, when mixed with oppositely charged counterparts, undergo phase separation. Traditionally, scientists believed they understood the basics: mixing oppositely charged polymers results in the formation of distinct phases, each enriched with specific components. However, grasping the finer details of how individual polyelectrolytes and their counterions distribute themselves within these phases has remained an elusive goal, limiting our ability to refine the materials built upon these interactions.
This gap in knowledge posed a significant challenge, especially given the immense potential of polyelectrolyte complexes in practical applications—ranging from water purification membranes to ion-exchange systems and bio-compatible materials. Without precise information on the distribution and behavior of all species involved, innovation remained somewhat of a trial-and-error process, slowing down progress.
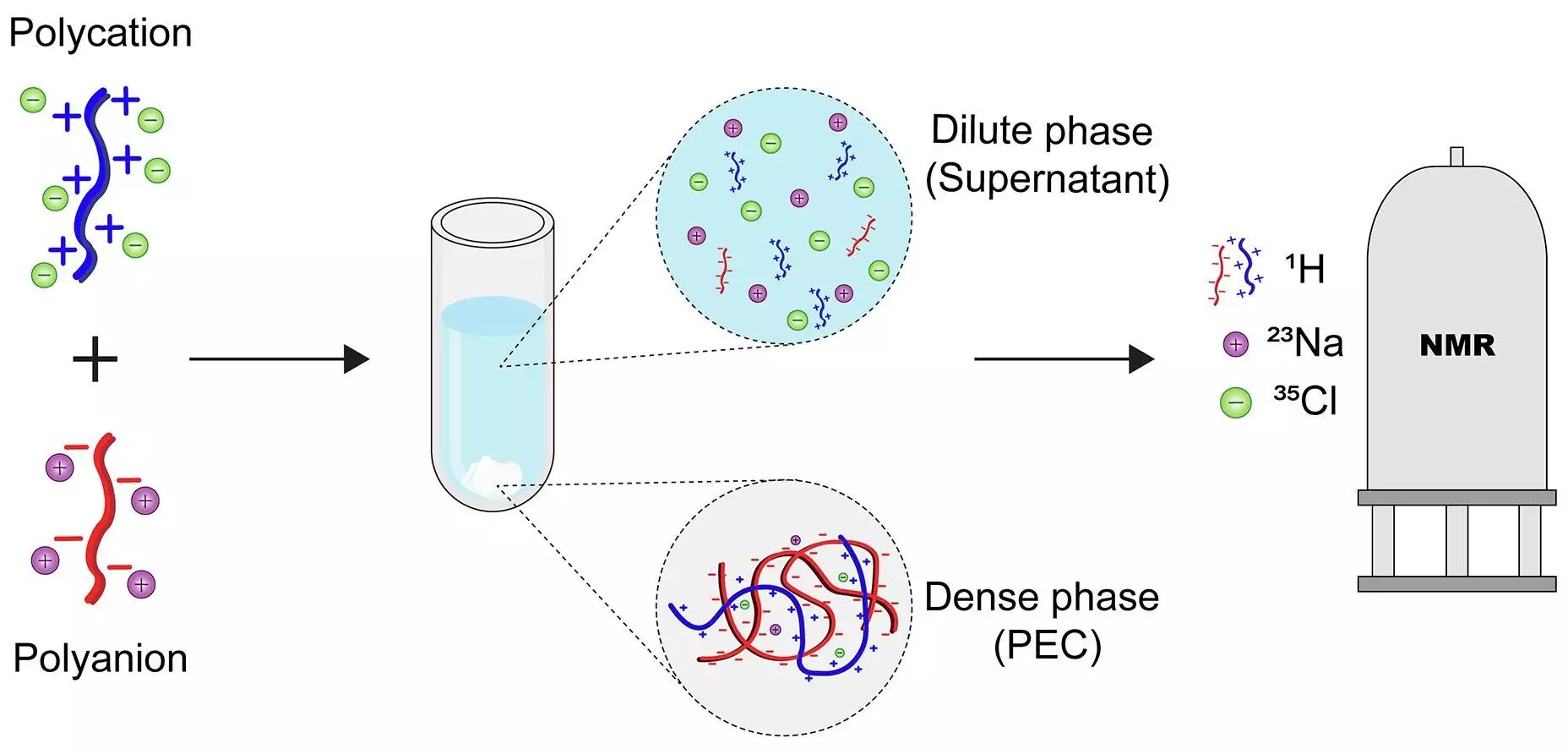
Innovative NMR Methodology: A Game Changer in Material Characterization
Enter the recent breakthrough from researchers at the University of Twente. By harnessing advanced Nuclear Magnetic Resonance (NMR) spectroscopy, these scientists have developed a method that pinpointedly measures the distribution of all components—both polymers and their associated counterions—in each phase after complexation. This is not merely a technical improvement; it’s a paradigm shift in how we study these intricate systems.
What makes this development remarkable is its speed and non-invasiveness. Achieving a complete analysis in under 40 minutes, the approach allows for rapid iteration and optimization of materials. Moreover, its label-free nature means it does not interfere with the system’s natural state, thus providing an authentic snapshot of the molecular landscape. Such capabilities are invaluable, considering that prior techniques struggled with either invasiveness, complexity, or lacked comprehensive quantification.
By providing a detailed thermodynamic and compositional portrait, this methodology empowers scientists to comprehend the true nature of polyelectrolyte complexes—how they form, evolve, and can be tweaked for specific applications. It opens doors for targeted design of functional materials with improved efficiency and durability.
Implications for Future Materials and Natural Interactions
This innovative approach doesn’t just serve the realm of synthetic material development. It holds the potential to deepen our understanding of naturally occurring polyelectrolyte systems—like those found in biological tissues and cellular matrices. Such insights could catalyze advancements in biomedicine, regenerative medicine, and environmental science.
From a practical perspective, mastering the precise component distribution can significantly enhance the performance of filtration membranes, therapeutic delivery systems, and recyclable materials. It invites a future where material scientists are no longer limited by incomplete data but can instead craft bespoke polyelectrolyte complexes with confidence, knowing exactly how each component will behave.
While challenges remain—such as scaling the technique for industrial applications—the ingenuity behind this NMR-based approach marks a crucial step forward. It exemplifies how a fundamental understanding, achieved through cutting-edge technology, can accelerate innovation and create materials that better serve humanity’s needs. This isn’t just about refining chemistry; it’s about redefining what’s possible in material science.

Leave a Reply