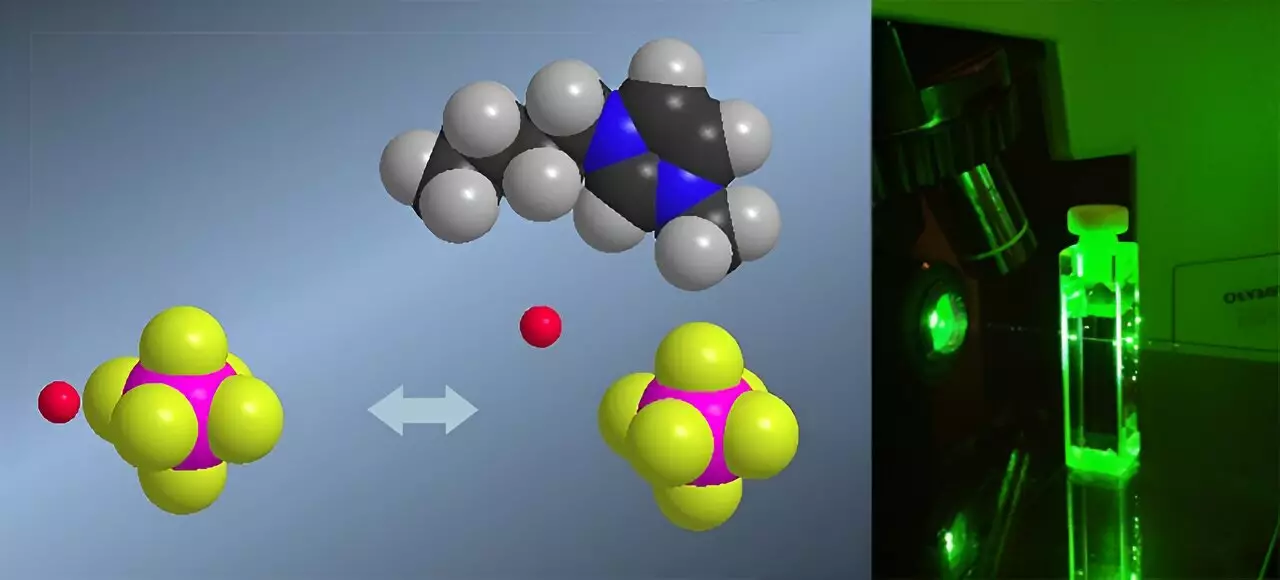
The scientific community has long grappled with the challenge of accurately measuring acidity in non-traditional solvents like ionic liquids. Conventional pH meters, reliable in aqueous solutions, falter when applied to these organic salts at room temperature. The breakthrough by researchers at the University of Liège introduces a novel approach: employing Raman spectroscopy to determine Hammett acidity functions. This development is not merely an incremental step but a potential paradigm shift, enabling scientists to explore reactant behaviors in complex, highly acidic environments with unprecedented precision.
What sets this method apart is its ability to circumvent the limitations of UV-visible spectroscopy, which demands optically transparent media and can be skewed by the presence of colored indicators. Raman spectroscopy’s compatibility with opaque and non-colored samples eliminates these hurdles, providing a clear window into the true acidity levels of ionic liquids. The significance of this innovation extends beyond mere measurement; it offers a new lens for understanding how protons behave in atypical media—insights that were previously obscured by technical constraints.
Understanding Super-Acidic Realms
One of the most astonishing revelations from this research is the confirmation that ionic liquids can exhibit acidity levels up to 100 million times greater than water. Such super-acidity defies traditional chemical intuition, as these solvents contain dissolved acids that are far more active than their aqueous counterparts. This extraordinary behavior arises from the unique structure of ionic liquids—they are composed of organic salts that do not readily solvate protons. Consequently, protons remain highly reactive, dramatically expanding the scope of acid-catalyzed phenomena accessible in such media.
The ability to measure this super-acidity directly is crucial for advancing applications across multiple fields. For instance, in catalysis, knowledge of exact acidity levels enables the design of more efficient and selective reactions. Similarly, in energy storage, understanding how ionic liquids behave as electrolytes can lead to the development of more stable batteries. Moreover, in biomass processing, accurate acidity measurements allow for optimized depolymerization processes—transforming waste into valuable resources.
Implications for Scientific and Industrial Innovation
The implications of this research extend well beyond academia. By successfully quantifying acidity in ionic liquids using Raman spectroscopy, scientists now have a powerful tool for both fundamental research and practical applications. This is particularly relevant in areas such as green chemistry, where ionic liquids serve as eco-friendly solvents that can replace hazardous chemicals. Precise acidity measurements could unlock new catalytic pathways, making processes more sustainable and cost-effective.
Furthermore, this technique bridges the gap between experimental and computational chemistry. Researchers can compare empirical data with theoretical models, refining their understanding of proton transfer mechanisms in complex solvents. Such synergy is vital for designing next-generation materials, from advanced batteries to environmentally friendly catalysts.
The work conducted at Liège is more than a technical achievement; it signifies a bold step toward harnessing the full potential of ionic liquids. By illuminating the enigmatic behavior of protons within these media, scientists are poised to unlock new realms of chemical innovation, with benefits that could ripple across industries and environmental sciences.

Leave a Reply