Recent advancements in organic chemistry have revealed the remarkable potential of quinolines as versatile feedstocks for the synthesis of complex organic molecules known as 2D/3D fused frameworks. These novel architectures are becoming pivotal in drug discovery and medicinal chemistry due to their intricate, customizable structures adorned with a variety of functional groups. The excitement surrounding quinolines stems from their unique electronic configuration; they contain an electron-rich benzene ring fused with an electron-poor pyridine ring. This distinct arrangement affords chemists the capability to selectively modify these rings under different reaction conditions, leading to a plethora of fascinating compounds.
The journey towards effective utilization of quinolines has not been without its challenges. Traditional approaches have predominantly focused on activating the benzene side of the molecule for syntheses, leaving the potentially rich chemistry offered by the pyridine ring largely unexplored. Until now, the full synthesis potential of quinoline derivatives remained a tantalizing yet elusive goal. Recent work from researchers in Japan is aiming to change that narrative.
A Game-Changing Methodology
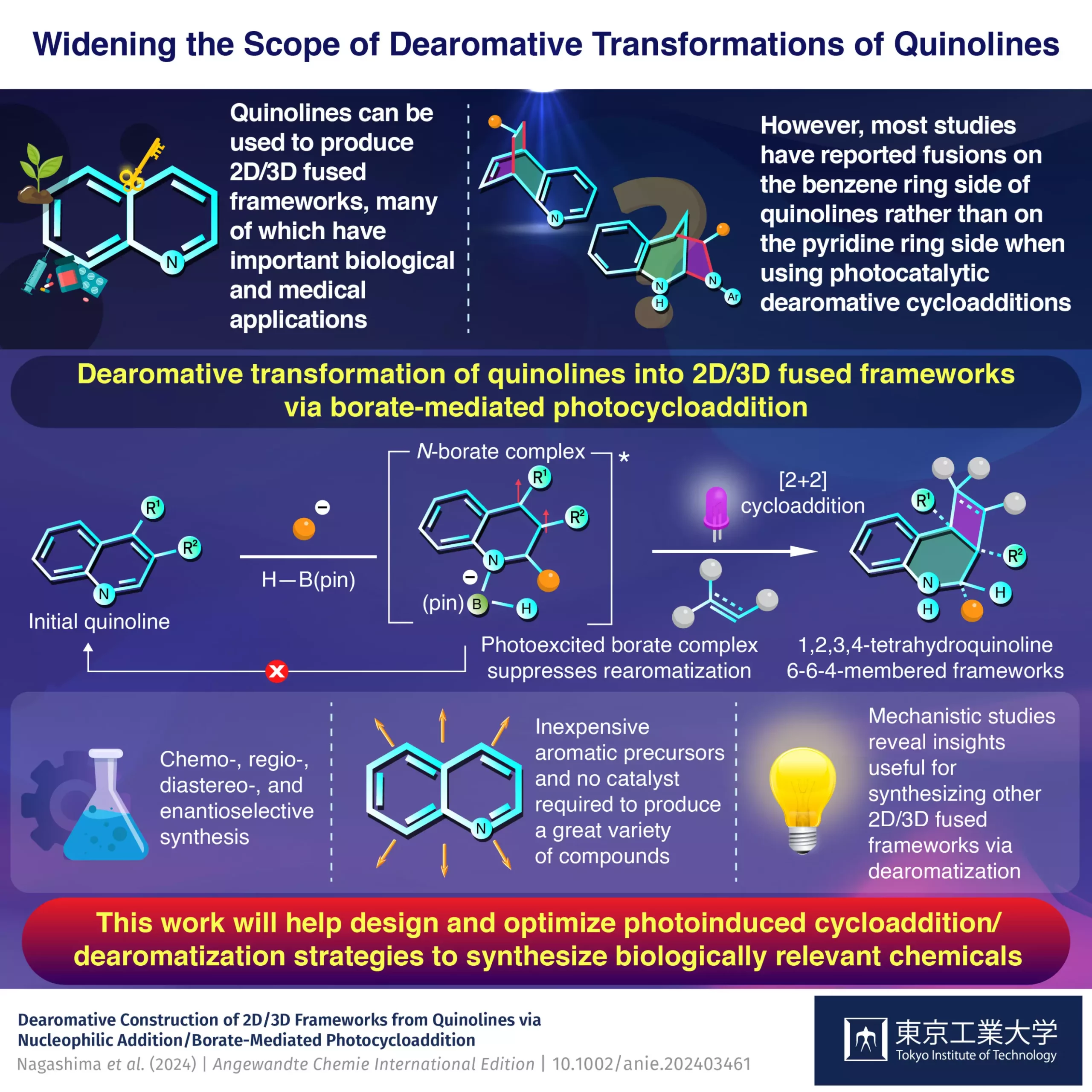
A team of scientists from the Tokyo Institute of Technology, spearheaded by Assistant Professor Yuki Nagashima, has devised an innovative and efficient strategy to access the pyridine side of quinolines and synthesize diverse 2D/3D frameworks. Their findings, published in the esteemed journal Angewandte Chemie International Edition, highlight a key molecule in this advancement—pinacolborane (H–B(pin)). This boron-containing gem has proven to be highly effective in facilitating dearomative photocycloadditions exclusively on the pyridine side of quinoline.
Through careful experimentation, Nagashima and his colleagues have unearthed a fascinating progression of events: the reaction begins with quinoline reacting with an organolithium compound, followed by a swift engagement with H–B(pin) to produce a crucial borate intermediate. This intermediate plays a pivotal role, as it not only accelerates the cycloaddition process but also prevents the typical rearomatization that interferes in conventional photocycloaddition reactions. The implications of this mechanistic understanding are profound, streamlining the synthesis and reducing the production of unwanted byproducts.
Advantages of the New Synthetic Approach
What sets this methodology apart from traditional synthesis routes is not just its efficiency but also its cost-effectiveness. By eliminating the need for catalysts and reducing the number of reaction steps, the researchers have significantly lowered the barriers for synthesizing complex compounds. This is especially beneficial in the pharmaceutical industry, where time and cost can heavily impact drug development timelines.
Furthermore, the flexibility of using multi-substituted starting materials means that chemists now have access to a versatile toolbox, enabling them to explore a wide variety of target compounds. This opens up gateways to previously unattainable molecular structures that could be pivotal in treating diseases. The adaptability of this technique is crucial for modern drug discovery, allowing researchers to rapidly prototype and screen potential candidates.
The Broader Impact on Organic Synthesis
The implications of Nagashima’s research extend well beyond the specific context of quinoline derivatives. Introducing boron-based photocycloadditions as a standard practice could redefine the landscape of organic synthesis, providing researchers with fresh avenues to explore the vast world of aromatic compounds. This study lays the groundwork for further innovations, possibly leading to the effective functionalization of various multi-ringed aromatic hydrocarbons.
As excitement builds around this breakthrough, the scientific community is already pondering its impact. The methodology could inspire a new wave of experiments that leverage similar principles across other classes of organic compounds. In a world where therapeutic needs continually evolve, the ability to create novel compounds efficiently could be a game-changer in addressing pressing medical challenges.
The fact that these transformations unlock a previously inaccessible realm of organoboron chemistry further elevates their importance, providing researchers with new tools to craft specialized molecules with exacting precision. As we stand on the precipice of this new frontier in organic synthesis, it is evident that the findings from Tokyo Tech are not merely a step forward; they represent a leap towards a future filled with possibilities in medicinal chemistry and beyond.

Leave a Reply