Gallium, the elusive metal first unveiled by French chemist Paul-Émile Lecoq de Boisbaudran in 1875, defies typical categorization in the periodic table. Renowned for its low melting point, gallium can famously transform from a solid into a liquid in mere seconds when placed in a warm beverage. However, recent research led by scientists at the University of Auckland reveals that gallium’s behaviors and structural attributes extend far beyond what was once understood. It is astonishing to think that after almost a century and a half, gallium continues to surprise researchers, unveiling complexities that could have profound implications for the future of materials science.
The Conflict with Long-Standing Assumptions
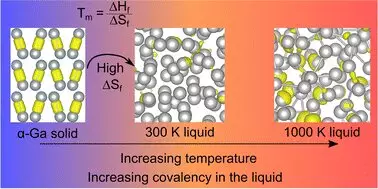
The recently published study in *Materials Horizons* titled “Resolving Decades of Debate: The Surprising Role of High-Temperature Covalency in the Structure of Liquid Gallium” unearths contrary evidence about gallium’s structural properties at various temperatures. Traditionally, researchers believed that the covalent bonds, which characterize gallium’s behavior and interaction at lower temperatures, vanished at the melting point, creating a seamless transition into a more disorderly liquid state. However, the team led by Dr. Steph Lambie has found that these bonds re-emerge at elevated temperatures, stirring up significant debate in the scientific community. Professor Nicola Gaston highlights a critical flaw in prior literature, indicating a misinterpretation that has persisted for over thirty years. This new insight doesn’t merely challenge established ideas; it compels scientists to reconsider the foundational principles driving understanding of metallic behaviors.
Entropy: The Key to Understanding Gallium’s Melting Point?
At the heart of this enlightening study is the element of entropy—a measure of disorder typically overlooked in discussions of metallic structures. The researchers suggest that the abrupt increase in entropy when gallium transitions from solid to liquid may provide a deeper understanding of its low melting point. As the covalent bonds dissolve, atoms gain freedom, resulting in a chaotic yet essential dance necessary for the unique properties of gallium. This revelation is not simply an academic curiosity; it is pivotal for advancing technologies in which precise control over material properties is crucial.
Applications and Future Prospects
The potential applications for gallium are as broad as they are fascinating. From the telecommunications sector to advanced semiconductor technology, gallium serves as a linchpin in countless high-tech industries. Its role in liquid metal catalysts and self-assembling structures is especially noteworthy, where it can boost efficiencies in processes ranging from chemical reactions to the creation of novel materials. Remarkably, past collaborative projects, which crystallized zinc in liquid gallium, showcased the unmatched versatility of this metal, hinting at broader implications for nanotechnology.
Further, gallium is being eyed not just on Earth but even beyond, as researchers propose it could reveal traces of past microbial life on Mars. This integration of materials science with astrobiology illustrates gallium’s potential to bridge knowledge gaps, driving both industrial and scientific innovation.
The Legacy of Discovery and Future Research Directions
The legacy of gallium is one of evolving mystery and continual exploration. Dmitri Mendeleev’s foresight in reserving a place in the periodic table for gallium speaks volumes about the potential scientific inquiries still waiting to be unleashed. The ongoing research at universities like Auckland and Victoria hints at a rich future of discovery. As gallium finds itself at the intersection of various disciplines—from computer sciences and nanotechnology to environmental research—its story is far from over.
With the scientific community energized by these new revelations and the implications they harbor, it will be exciting to witness how gallium continues to enamor and inspire advancements in materials science and beyond. The dialogue surrounding its properties is only beginning, and with each discovery, gallium proves to be not just a metal but a key to unlocking unexplored territories in science and technology.

Leave a Reply