Ice, while seemingly a solid and impenetrable substance, is frequently enveloped by liquid water. This dynamic relationship serves as the key to countless natural phenomena—from winter sports to the formation of snowflakes and even the enjoyable experience of ice cream. A pivotal study conducted by researchers at Kobe University and the Institute for Molecular Science takes us closer to understanding this complex interaction by investigating the interface between ice and liquid at an unprecedented level. This exploration is not just an academic exercise; it brings forth insights that resonate with both nature appreciation and scientific innovation.
Innovative Methodology: Observing Ice in Antifreeze
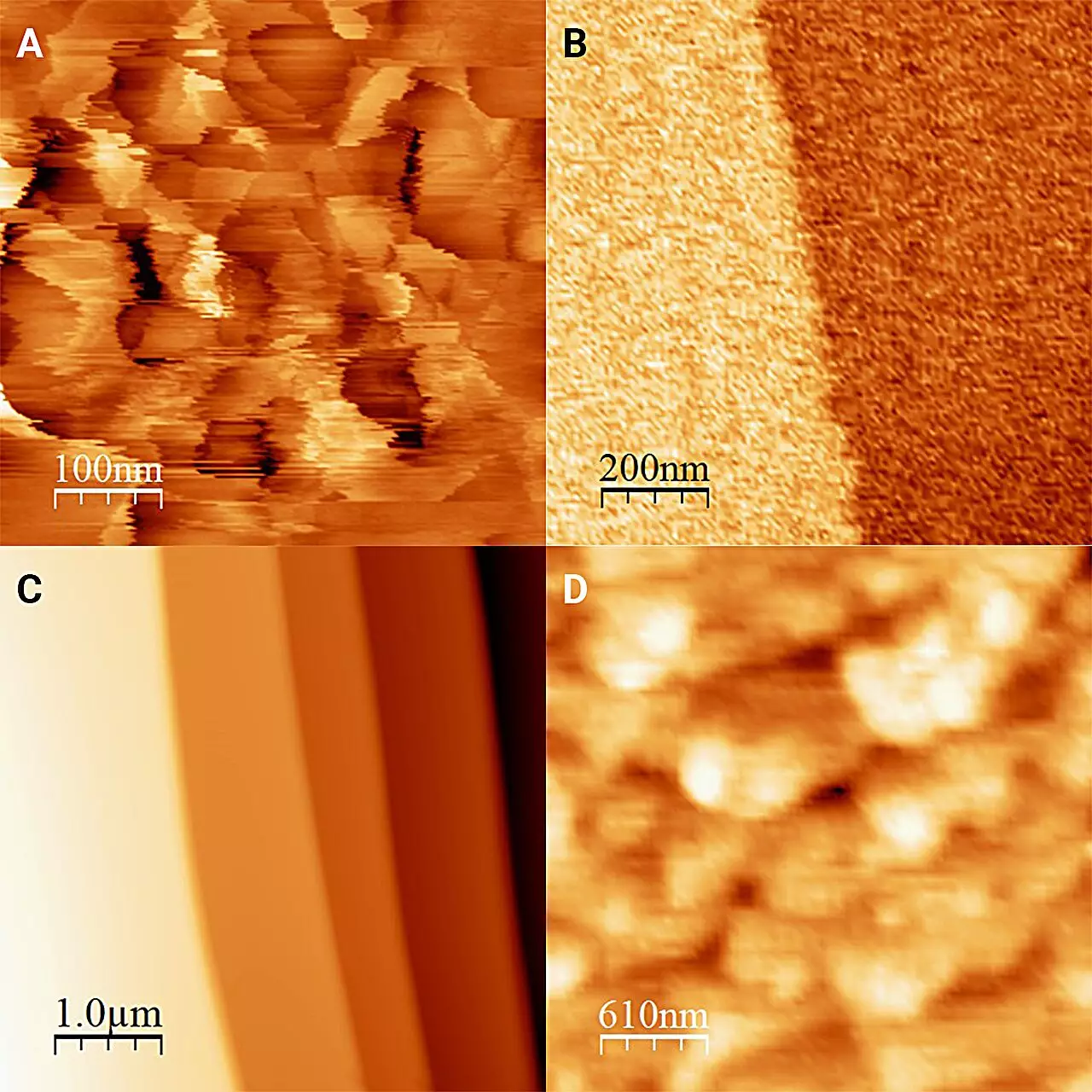
Research in the realm of ice and water has traditionally faced significant hurdles due to the rapid interconversion of these two states of matter. Ice can quickly melt into water, creating a continuously changing interface that makes direct observations nearly impossible. To combat this challenge, the researchers, led by Onishi Hiroshi, devised a clever strategy: isolating the ice within antifreeze at sub-zero temperatures. This ingenious approach allowed them to maintain a stable ice form without the interference of melting, effectively freezing the dynamics enough for accurate observation.
Through relentless experimentation, the researchers equipped their atomic force microscope—a highly sensitive instrument—inside a cooled box, allowing it to function optimally in a chilling environment. This meticulous preparation was critical not just for the clarity of observation, but also for validating the methods used. The results of their labor were ultimately published in The Journal of Chemical Physics, shedding light on the previously obscured relationship between ice and its liquid counterpart.
Key Findings: The Shape and Hardness of Ice at Interface
One of the striking revelations from this study is the notable difference in the structure of ice when surrounded by liquid as opposed to isolated air. While conventional ice displays “frost pillars” measuring approximately 20 nanometers in height, their findings under antifreeze revealed an astonishingly flat ice surface punctuated only by minuscule steps measuring a single molecular layer. The researchers attribute this alteration in structure to a process of partial dissolution and recrystallization induced by the liquid environment.
Furthermore, they examined the surface hardness of ice treated with 1-octanol—an alcohol used as an antifreeze in this study—and found it to be considerably harder than what earlier methods had suggested. This finding opens new avenues for understanding ice’s physical properties and has implications for fields such as glaciology, meteorology, and material science.
The Implications of a Better Understanding of Ice
The research prompted by Onishi and his team does not merely represent a milestone in micro-interactions between ice and liquid, but it also beckons a broader consideration of how these findings could transform scientific inquiries. Understanding ice at such a minute level not only expands our fundamental knowledge but also has practical implications. For instance, enhancing our grasp of how ice behaves in both natural and controlled environments can affect weather prediction models, climate change studies, and even industries reliant on cold chains.
Moreover, the researchers have not settled for their current findings. They are clearly ambitious, expressing intent to elevate their observation capabilities to the point where they can measure interactions at the scale of single water molecules. This forward-thinking approach promises to refine our understanding further and challenges future researchers to innovate beyond what has already been accomplished.
In the end, this study represents a beautiful convergence of creativity, dedication, and scientific rigor. It frames ice not merely as a winter inconvenience but as a complex material whose properties still hold many secrets. Through innovative techniques and a relentless pursuit of knowledge, we inch closer to unraveling the intricate dance between ice and liquid, redefining our relationship with this elemental force.

Leave a Reply